Use of exosomes for the treatment of disease
a technology of exosomes and disease, applied in the field of exosomes, can solve problems such as antibody disruption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Method used
Image
Examples
example 1
r Properties of Inhibitory RNA-Containing Exosomes
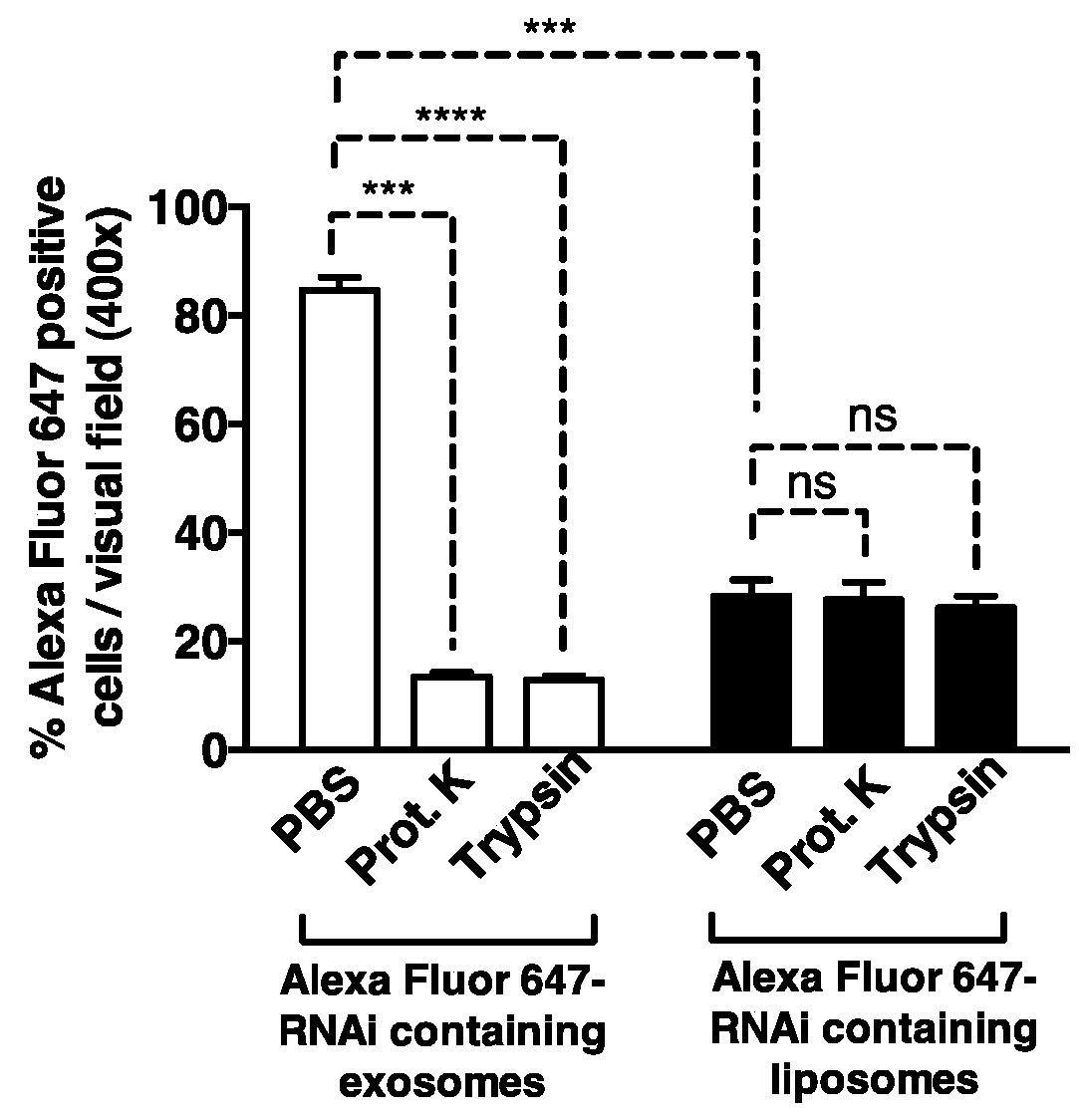
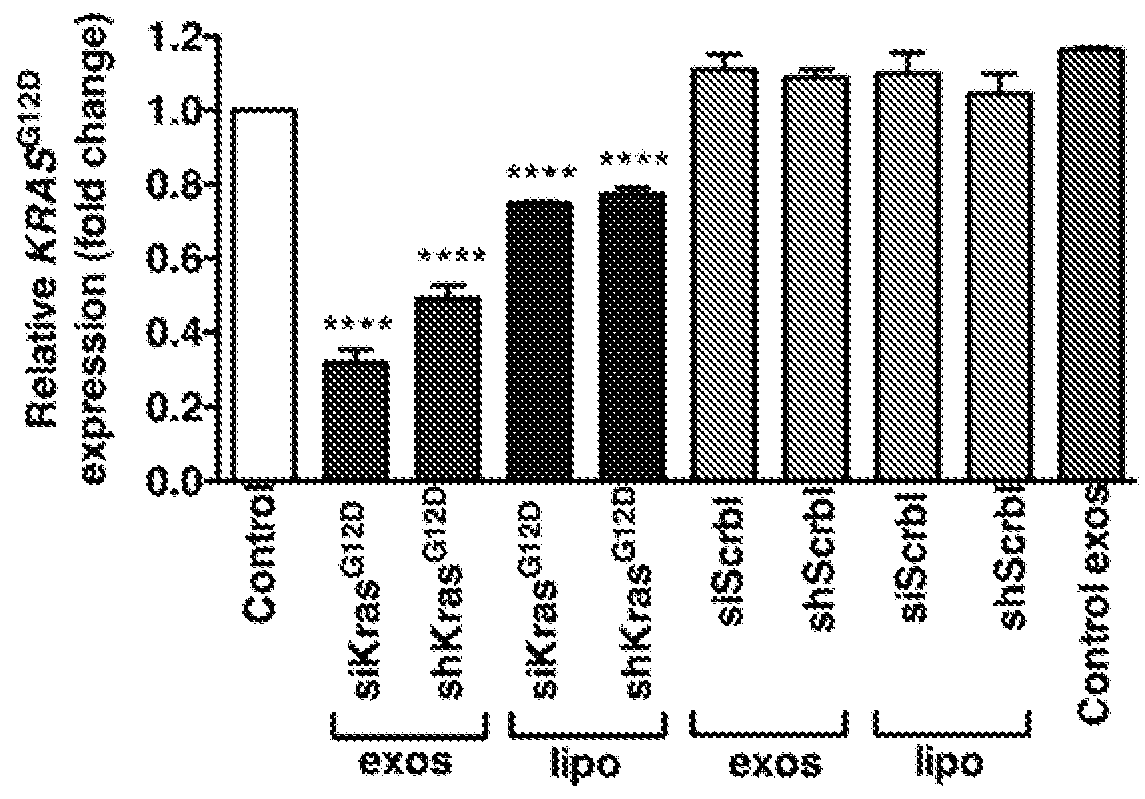
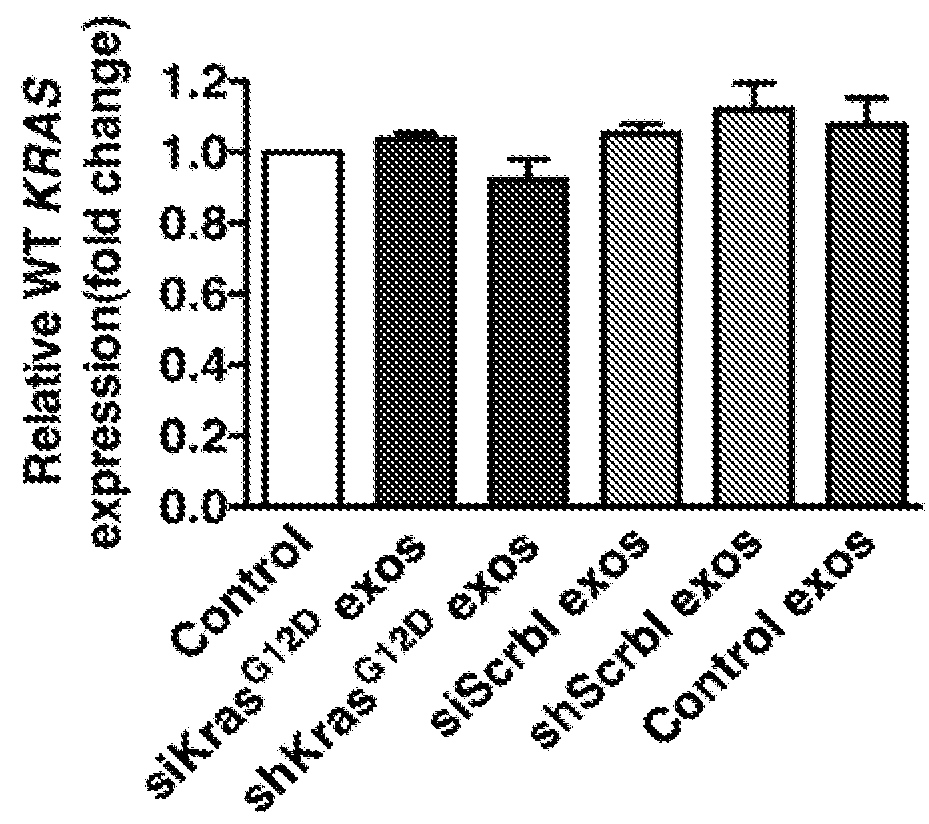
[0213]siRNA and shRNA constructs were designed to specifically target KrasG12D. The siRNA sequence (GUUGGAGCUGAUGGCGUAGTT; SEQ ID NO: 1) reflects a G to A nucleotide deviation from the wild-type Kras gene sequence (underlined and bold) so as to specifically target the Glycine to Aspartate amino acid substitution in the KrasG12D mutation found in cell lines and animal models, and a TT nucleotide overhang (underlined) to promote silencing efficiency (Rejiba et al., 2007; Ma et al., 2004; Du et al., 2005). The central position of the nucleotide deviant in this KrasG12D siRNA enhances its specificity against the wild-type mRNA sequence (Du et al., 2005). The shRNA sequence (SEQ ID NO: 2) was designed to contain the specific G to A nucleotide deviation in the seed sequence to promote the specific targeting of KrasG12D mRNA. The siRNA oligonucleotides for KrasG12D were also labeled with an Alexa Fluor® 647 fluorophore to track their delive...
example 2
ents Uptake of Exosomes by Circulating Monocytes
[0217]Circulating monocytes were found to engulf liposomes (100 nm; purchased from Encapsula Nanosciences) but not exosomes (FIGS. 8A-8B). Exosomes isolated from BJ fibroblasts were found to comprise CD47 on their surface (FIGS. 9A and 9C) while liposomes were determined to lack CD47 on their surface (FIG. 9B). Treatment of exosomes with an anti-CD47 antibody was found to stimulate the uptake of exosomes by circulating monocytes in vivo (FIG. 10).
[0218]All of the methods disclosed and claimed herein can be made and executed without undue experimentation in light of the present disclosure. While the compositions and methods of this invention have been described in terms of preferred embodiments, it will be apparent to those of skill in the art that variations may be applied to the methods and in the steps or in the sequence of steps of the method described herein without departing from the concept, spirit and scope of the invention. Mor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Real time PCR | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com