Methods and compositions for treating cancers using antisense
a cancer and antisense technology, applied in the field of compositions and methods for treating cancers using antisense, can solve the problems of poor prognosis of malignant glioma, particularly glioblastoma multiforme, and the inability to address the challenges created by solid tumors in immunotherapy trials, and achieve optimal anti-tumor response and inhibit tumor regrowth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0134]Vaccination with Autologous Tumor Cells and IGF-1R AS ODN in Patients with Recurrent Glioblastoma
[0135]Criteria and Study Objective
[0136]Twelve subjects were enrolled for treatment after failure from standard therapy. Each patient met the following criteria: age>18, a Karnofsky performance score of 60 or better, and no co-morbidities that would preclude elective surgical re-resection. The subjects were treated by 24 hour implantation in the rectus sheath of ten biodiffusion chambers containing irradiated autologous tumor cells and IGF-1R AS ODN with the objective of stimulating tumor immunity. Patients were monitored for safety, clinical and radiographic as well as immune responses. Study objectives included assessment of safety and radiographic responses as well as exploratory objectives looking at immune function and response.
TABLE 1Summary of Patients enrolledTimeOriginalLymphocyteIDH-1&IDH-2betweenlymphocytecount atmutation / surgeriesChamberscountenrollmentPreviousMGMTSubje...
example 2
[0185]Vaccination of Newly Diagnosed Subjects with Glioblastoma
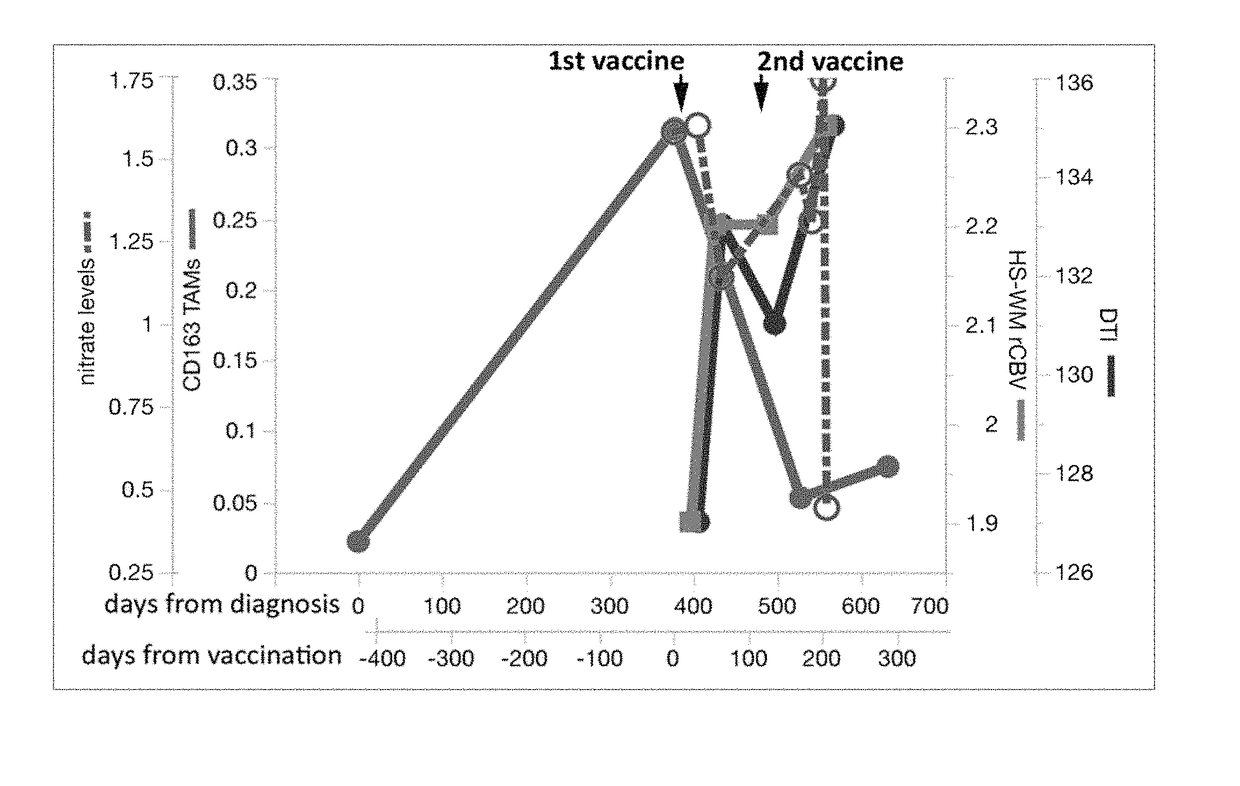
[0186]We demonstrated biological effectiveness of the vaccine protocol involving an autologous cell vaccine delivered as part of a formulated combination product involving implanted biodiffusion chambers in patients with recurrent malignant gliomas who had failed standard treatment,
[0187]Example 2 describes responses to administering the vaccine to newly diagnosed glioma patients, including implanting 20 chambers for 24 or 48 hours and 10 chambers for 24 or 48 hours. In each case, 2 μg of NOBEL was added into the chamber prior to irradiation in each case. When compared to standard of care in the first interim analysis, there were significant improvements in both progression-free survival and overall survival (FIG. 9). This was most notably due to the performance of the higher dose cohorts after vaccination. We first noted significantly higher peak and mean interferon-gamma levels after vaccination in the newly diagnosed ...
example 3
Fully Formulated Chambers Have Greater Adjuvanticity
[0189]The fully formulated chamber includes the autologous tumor cells and other cells included in the tumor microenvironment (TME) treated 6 hours prior to implantation with 4 mg / ml of IGF-1R AS ODN. The treated TME is then encapsulated with exogenous addition of at least 2 μg of IGF-1R AS ODN and the chamber is then irradiated with 5 Gy of gamma-irradiation.
[0190]We increased the number of chambers, meaning that the dose of IGF-1R AS ODN received by each patient increased compared to previous studies. For example, twice the number of chambers implanted resulted in twice the amount of the antisense implanted and capable of diffusing out of the chamber, meaning the dose of AS ODN was about 40 μg, split between 20 chambers.
[0191]The antisense sequence, particularly its palindromic CpG motif, and the direct mixture with glioma cells in situ effectively initiate anti-tumor immunity. Notably, the sense sequence with the same palindromi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| wet weight yield | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com