Optimized Anti-CD3 Bispecific Antibodies and Uses Thereof
a technology of bispecific antibodies and anti-cd3 is applied in the field of bispecific antibodies targeting effector antigens, which can solve the problems of inconsistent bsabs, difficult to predict differential outcomes, and high price of bsabs, and achieves reduced clearance, high affinity, and weak affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
n of Anti-CD3 Antibodies
[0177]The following procedures were aimed at identifying antibodies that specifically recognized CD3 (T cell co-receptor) as an antigen.
[0178]A pool of anti-CD3 antibodies were derived by immunizing genetically modified mice. Briefly, mice genetically engineered to express reverse chimeric (human variable, mouse constant) and immunoglobulin heavy chains associated with a single rearranged light chain (e.g., a VK1-39 / J or a VK3-20 / J), were immunized with a CD3 antigen and generated B cells that comprised a diversity of human VH rearrangements in order to express a diverse repertoire of high-affinity antigen-specific antibodies. Certain exemplified antibodies described in the subject application have been made recombinantly and express the same light chain sequence of VK1-39Jκ5 (LCVR set forth in SEQ ID NO: 162), while other antibodies made recombinantly express a cognate light chain of one of the heavy chain arms (e.g. the tumor target arm).
[0179]Generated ant...
example 2
Light Chain Variable Regions (Amino Acid and Nucleic Acid Sequences of the CDRs)
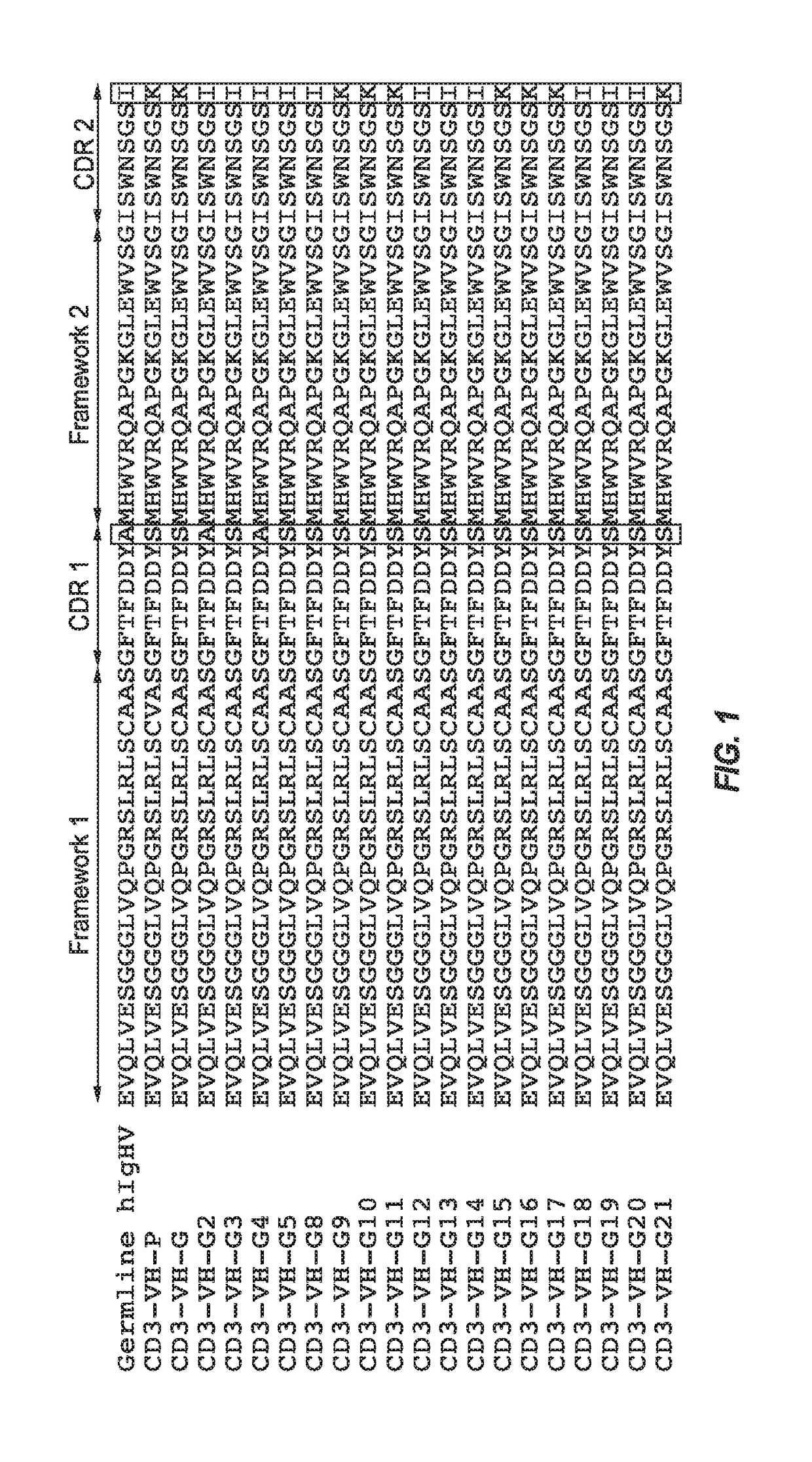
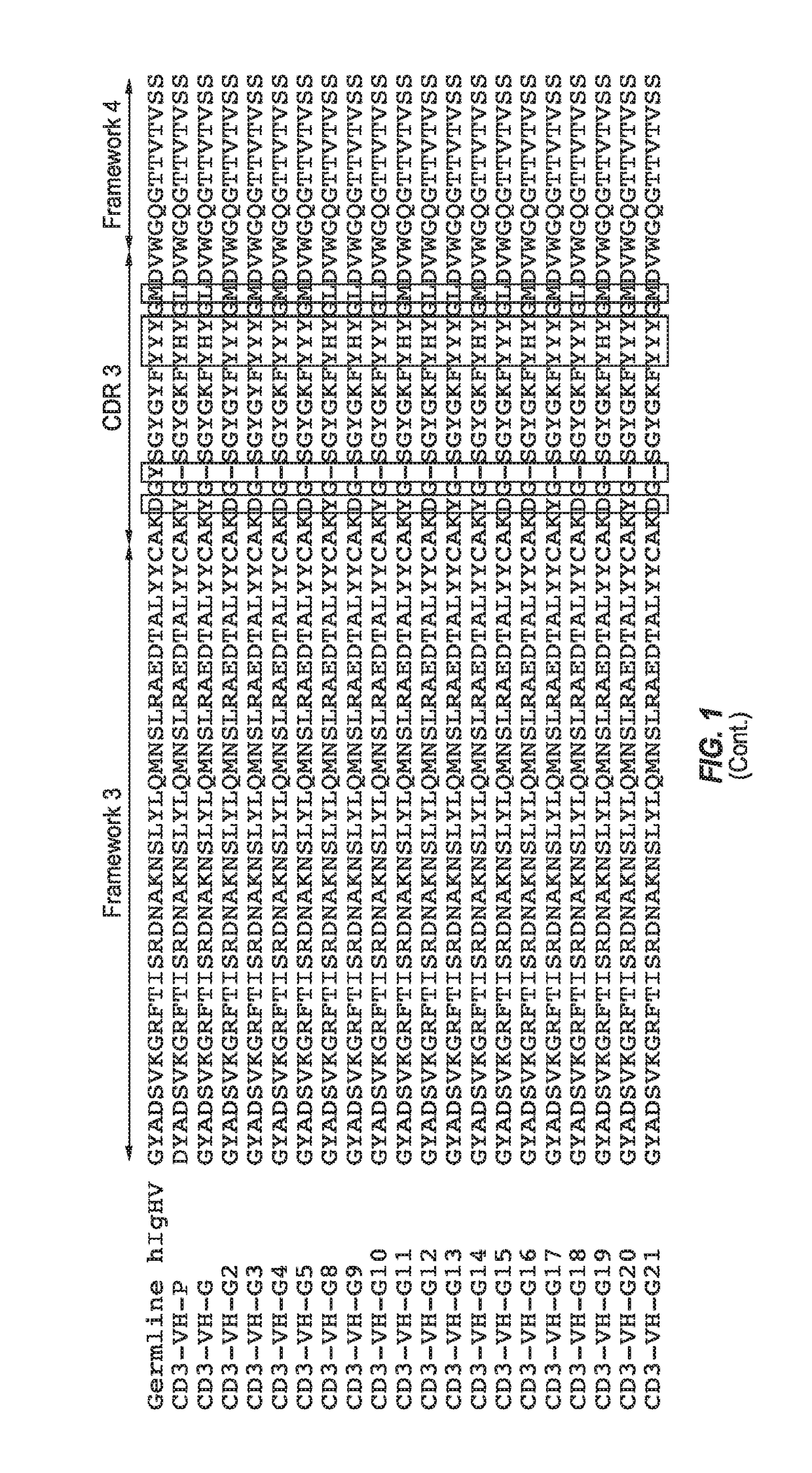
[0183]Amino acid and nucleic acid sequences were determined for each antibody heavy chain sequence. Each antibody heavy chain, as a derivative of the germline sequence IGHV3-9*01 / D5-12*01 / J6*02 (SEQ ID NO: 181) was assigned a “G” number designation for consistent nomenclature. Table 2 sets forth the amino acid sequence identifiers of the heavy chain variable regions and CDRs of the engineered anti-CD3 antibodies of the invention. The corresponding nucleic acid sequence identifiers are set forth in Table 3. The amino acid and nucleic acid sequence identifiers of the light chain variable region and CDRs to construct each recombinant antibody are also identified below in Tables 4 and 5, respectively.
TABLE 2Heavy Chain Amino Acid Sequence IdentifiersAntibodyCD3-VHSEQ ID NOs:DesignationHCVRCDR1CDR2CDR3CD3-VH-G2468CD3-VH-G210121416CD3-VH-G318202224CD3-VH-G426283032CD3-VH-G534363840CD3-VH-G842444648CD3-VH-G9505...
example 3
n of ULC Bispecific Antibodies that Bind CD3 and Tumor-Associated Antigens (TAA)
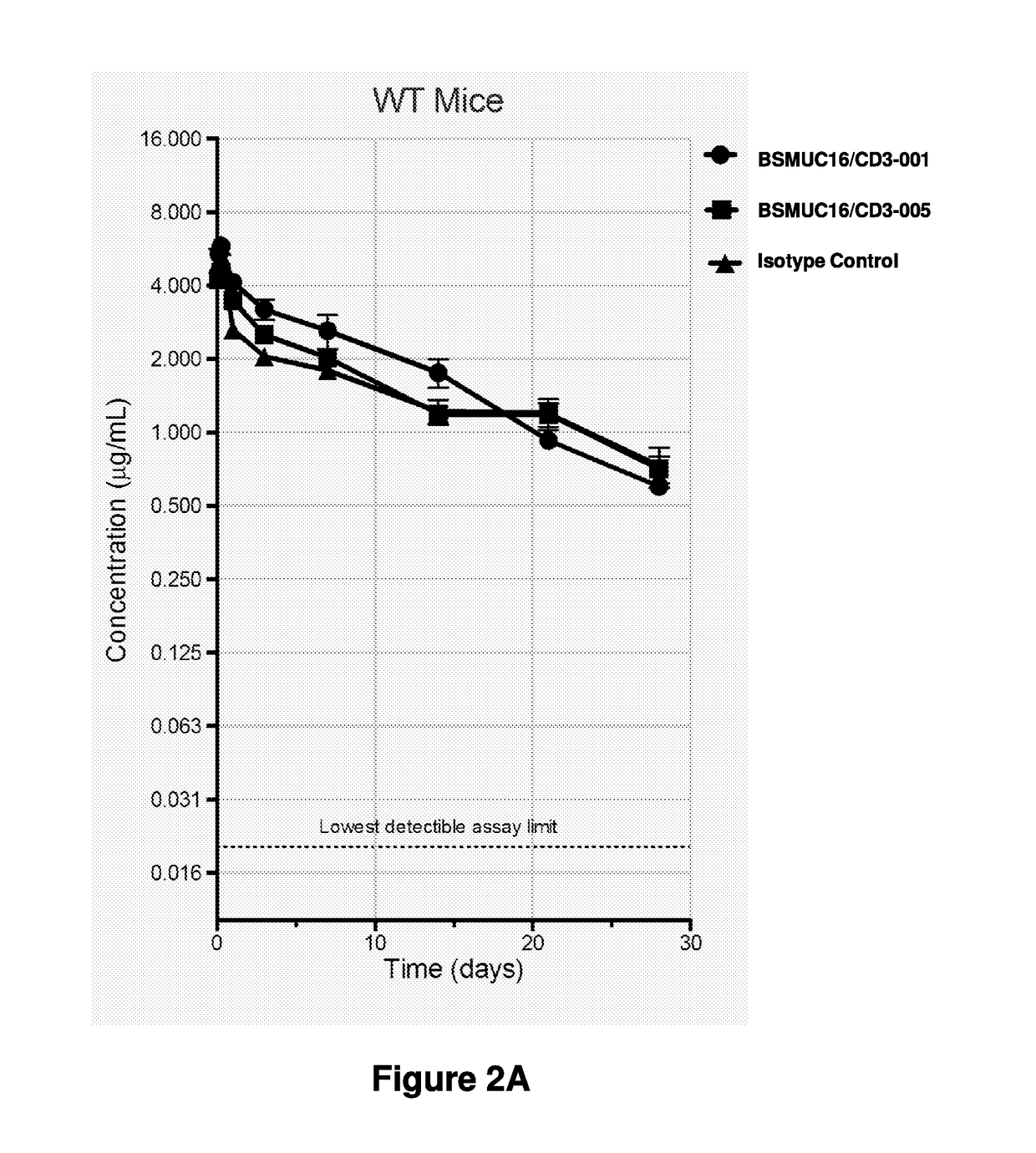
[0186]Bispecific antibodies comprising an anti-CD3-specific binding domain and an anti-TAA-specific binding domain, such as PSMA, EGFRvIII, MUC16, or STEAP2, were constructed using standard molecular biology methodologies utilizing a heavy chain from an anti-CD3 antibody described herein, a heavy chain from an anti-TAA antibody and a common light chain or a universal light chain (ULC). The anti-TAA antibodies used to construct the bispecific antibodies of this invention were obtained by immunizing genetically modified mice.
[0187]A summary of the component parts of the antigen-binding domains of the various bispecific antibodies made in accordance with this Example is set forth below in Tables 6, 7 and 8. All bispecific antibodies were manufactured having a modified (chimeric) IgG4 Fc domain as set forth in US Patent Application Publication No. US20140243504A1, published on Aug. 28, 2014. Exemplary EGFRvI...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com