Methods of treating chronic disorders with complement inhibitors
a technology of complement inhibitors and chronic disorders, applied in the field of chronic disorders with complement inhibitors, can solve the problems of 300 million individuals worldwide, morbidity and mortality whose incidence is increasing worldwide, asthma is also a significant global health problem, etc., and achieves the effects of inhibiting plasma complement activation capacity, prolonging the effect, and reducing one or more manifestations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
a Potent Compstatin Analog in Ascaris suum Animal Model of Asthma
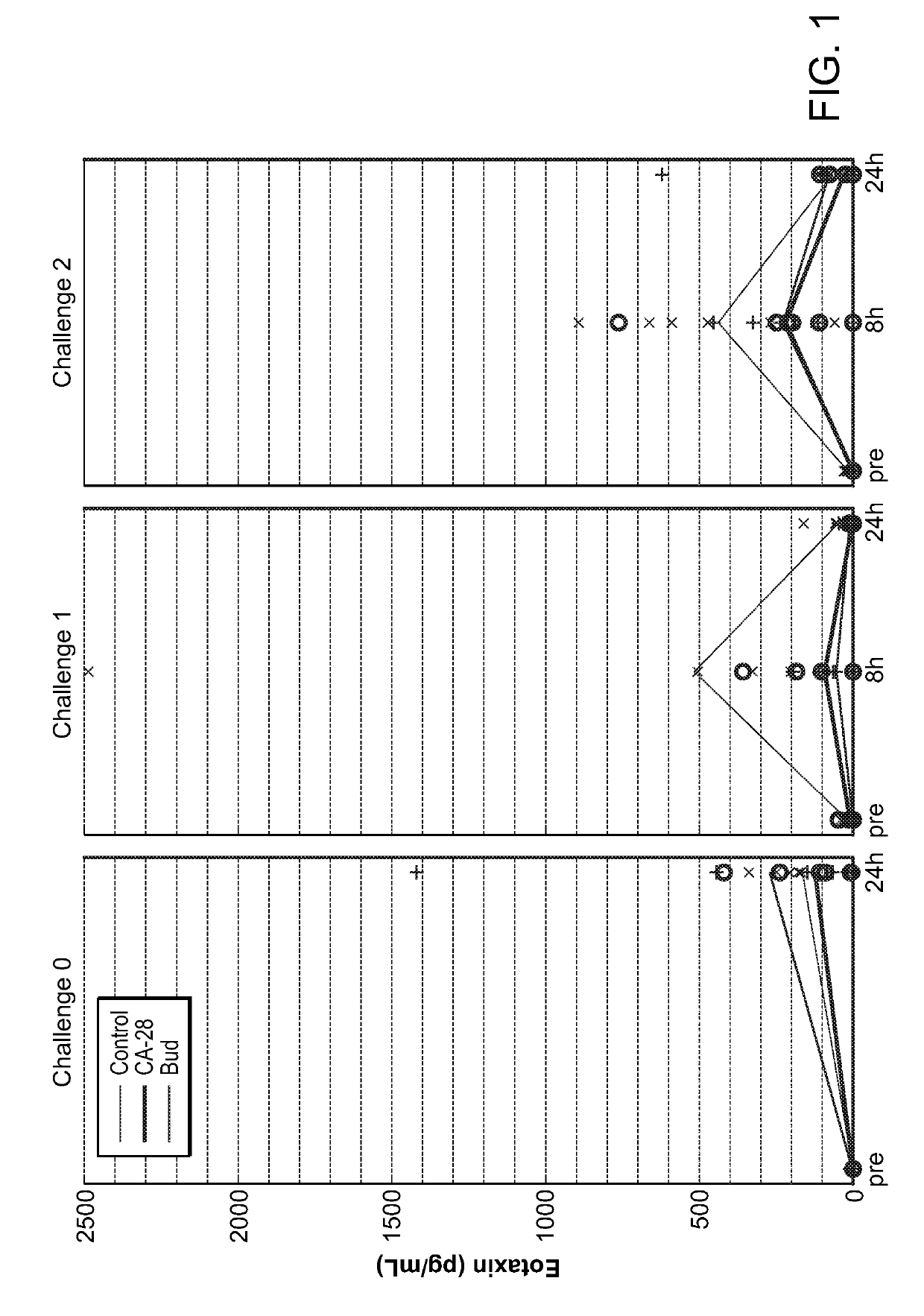
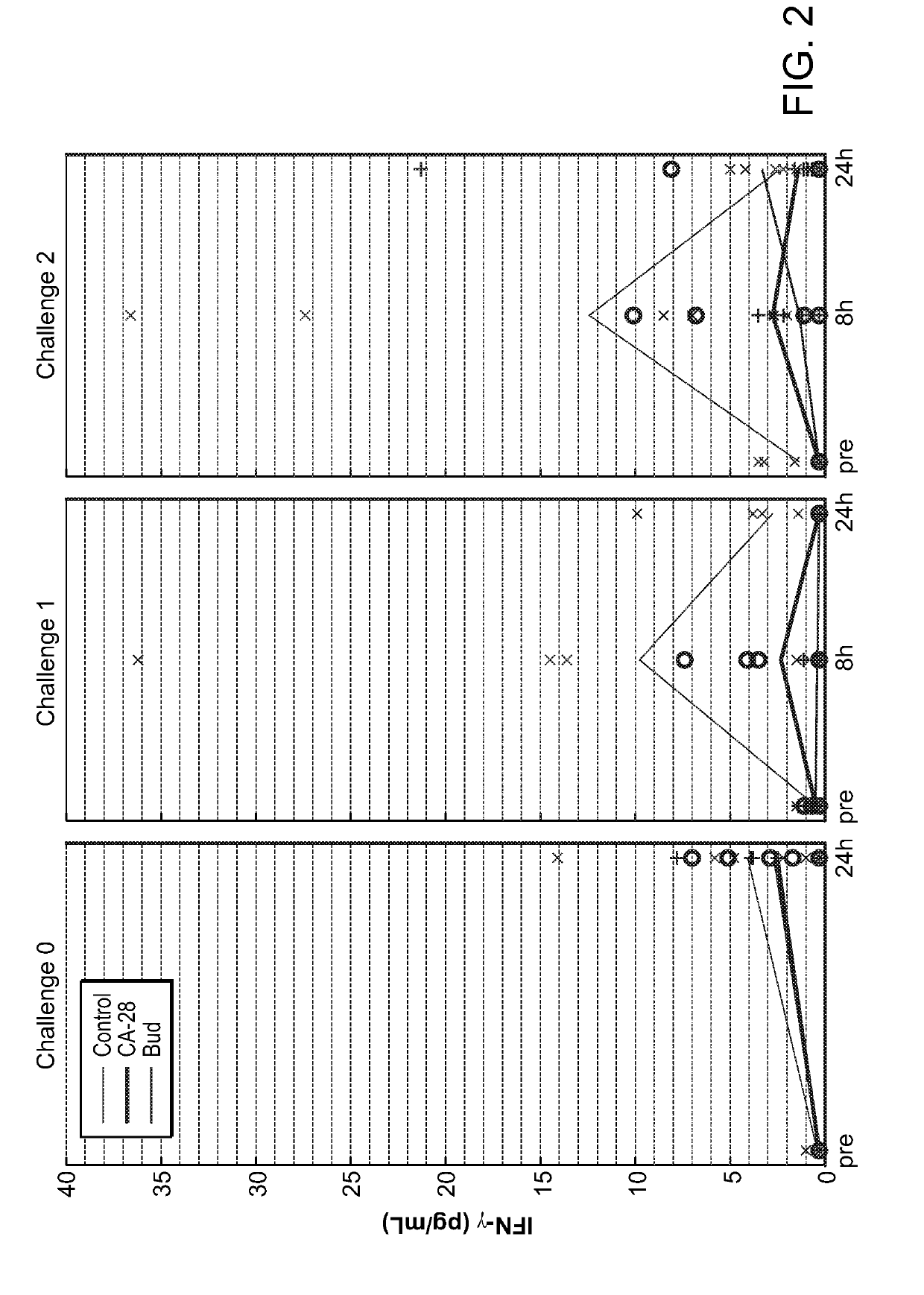
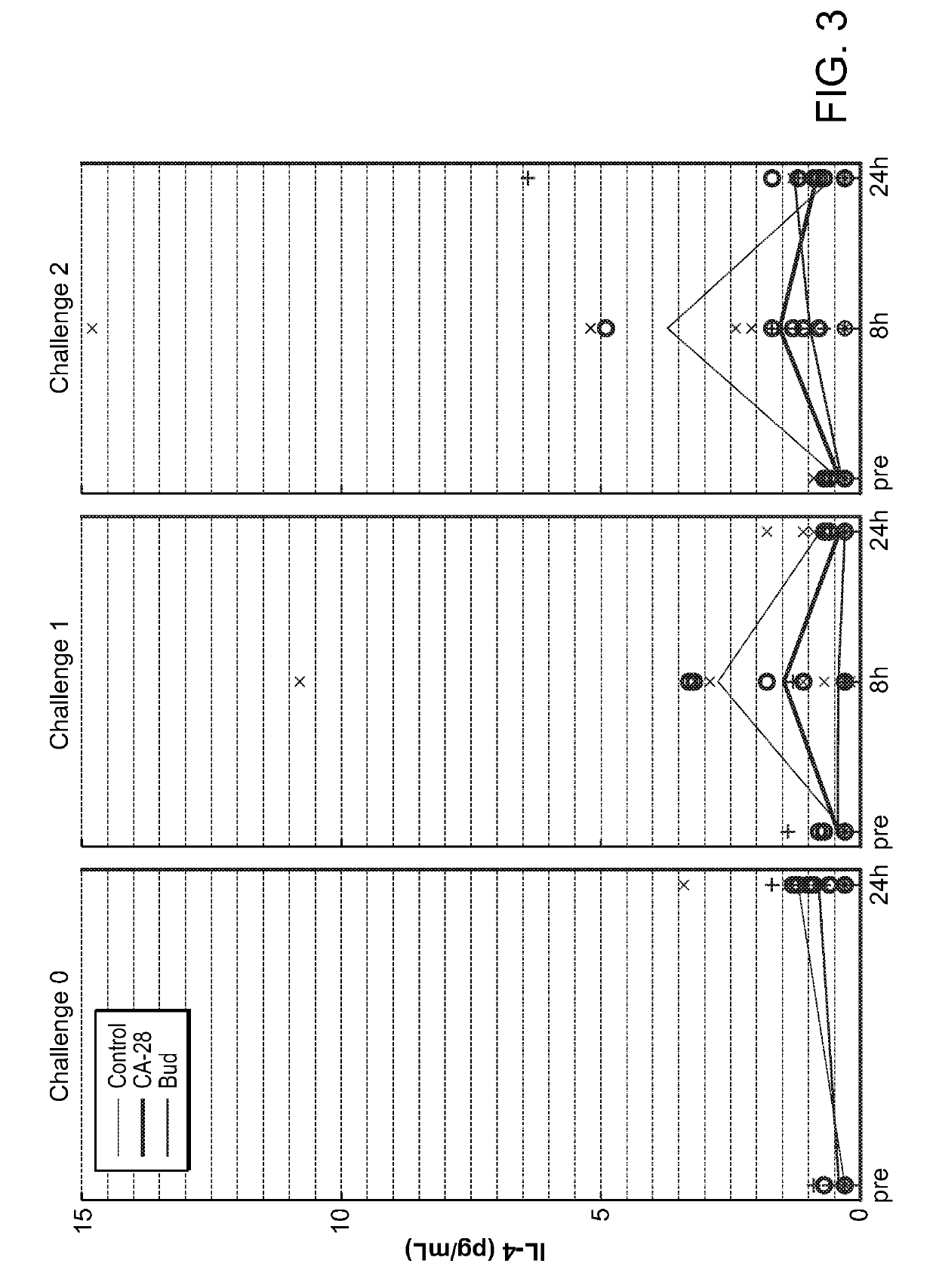
[0359]A potent compstatin analog having the amino acid sequence of the compstatin analog of SEQ ID NO: 28, was synthesized using standard methods. Briefly, amino acids were obtained as Fmoc-protected amino acids, in which the α-amino group of each amino acid was protected with Fmoc. Side chain functional groups were also blocked with various appropriate protective groups. Synthesis was accomplished following the solid phase methodology described by Merrifield (J. Amer. Chem. Soc. 85, 2149 (1963)). Chain assembly was performed on solid phase, at the conclusion of which the N-terminus was acetylated; the peptide was then cleaved from the solid phase and simultaneously deprotected via acidolysis using TFA and amidated. The linear peptide was then oxidized and purified. A study designed to evaluate the efficacy of CA-28 after 14 days of administration in a non-human primate model of asthma was performed. In this study, a d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com