Combination therapy for the treatment of autoimmune diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
Example 1
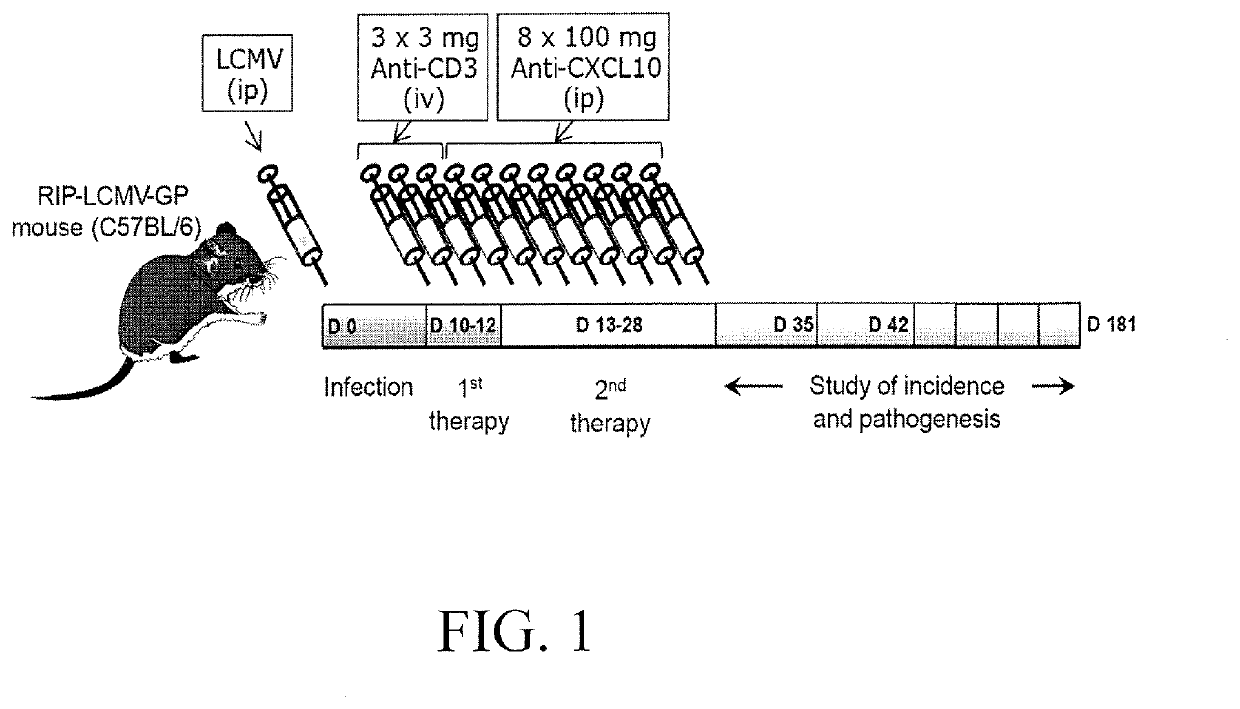
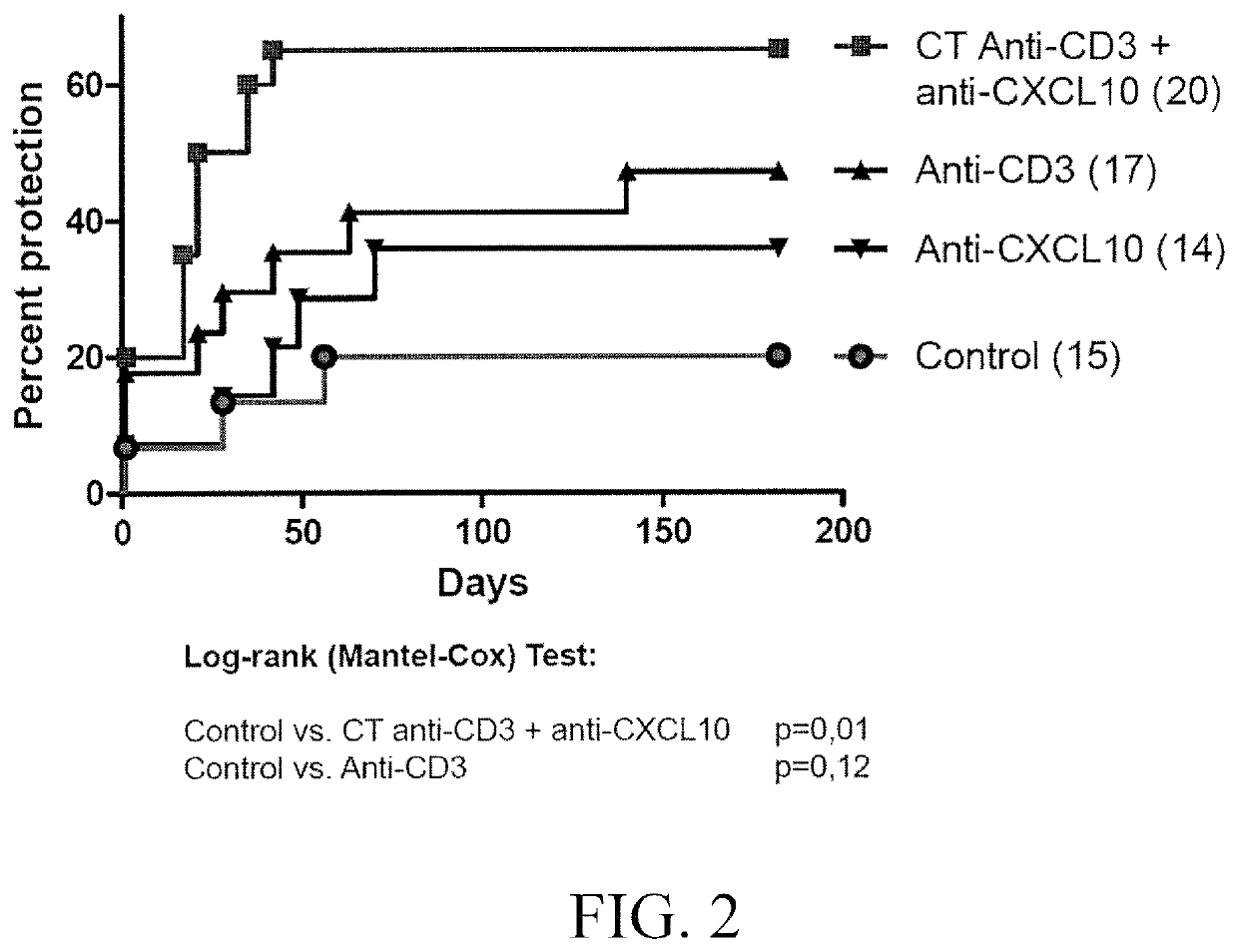
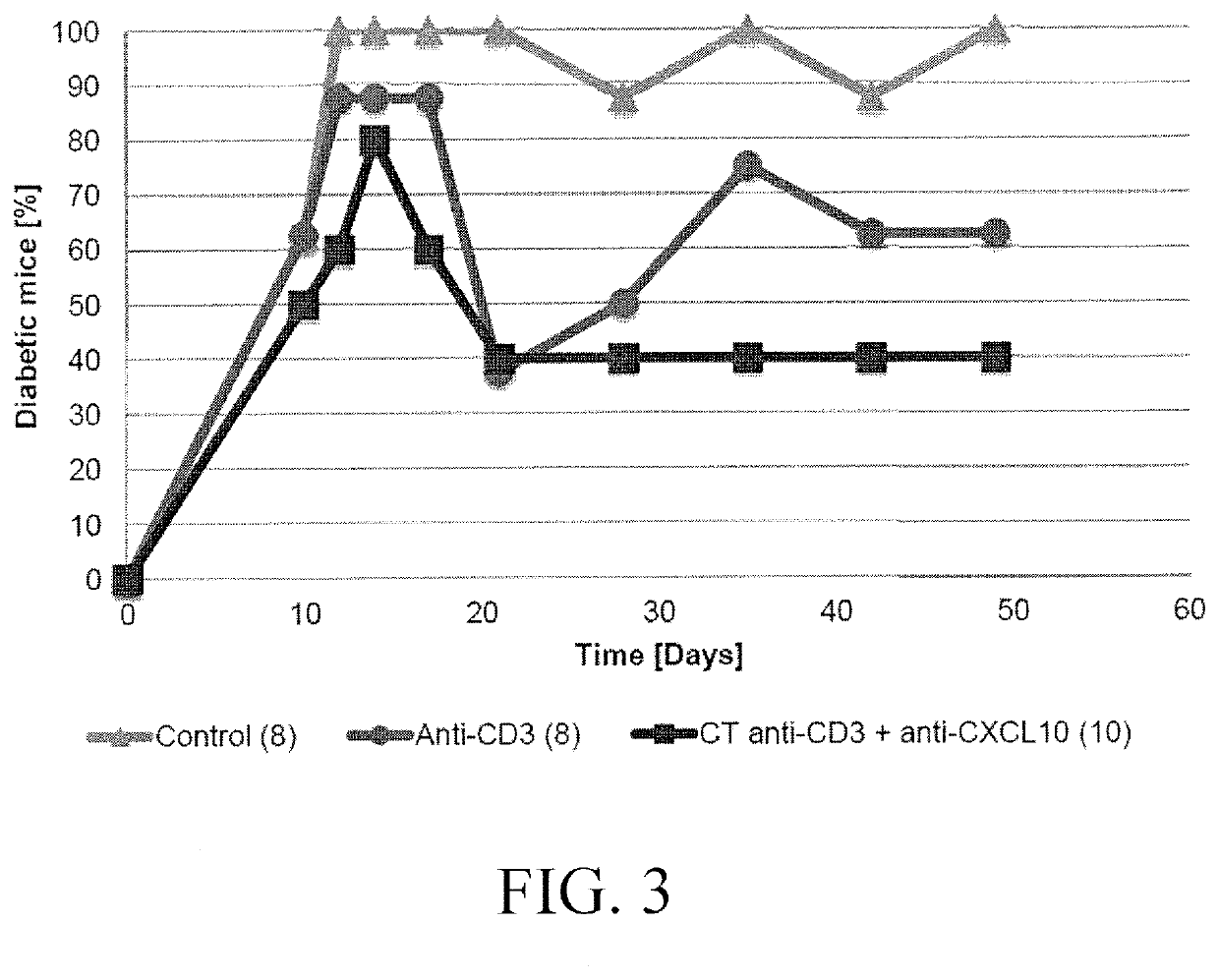
[0062]As a model for diabetes type 1 the RIP-LCMV mouse was used. Transgenic RIP-LCMV-GP mice express glycoprotein (GP) of the Lymphocytic Choriomeningitis Virus (LCMV) under the control of the rat insulin promoter (RIP). The promoter allows for the specific expression in the beta cells of the Langerhans islets of the pancreas (Oldstone MBA et al, Cell, 1991). The transgenic mice therefore express the viral GP and tolerate the protein as “self”. However, infection with the LCMV induces a LCMV specific immune response that not only targets the virus but also the beta cells expressing the viral GP protein. Usually, the RIP-LCMV-GP mice develop type 1 diabetes after 10 to 14 days of the infection.
[0063]For the experiments, the above mice when diabetic were treated for three days with 3 μg / day anti-CD3 antibody (Aimenian hamster anti-mouse CD3e IgG F(ab′), clone 145-2C11; Chatenoud L, et al. 1997, J Immunol.). Subsequently, the mice were treated three times a week with 100 μg a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Therapeutic | aaaaa | aaaaa |
| Inhibition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com