Method for treating pulmonary fibrosis using s100a3 protein
a pulmonary fibrosis and s100a3 technology, applied in the field of molecular genetics, diagnostic and therapeutic medicine, pharmacology, etc., to achieve the effect of restoring the normal calcium response and reducing the expression of the protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
[0072]The following Example illustrates various aspects of the present invention. They are not to be construed to limit the claims in any manner whatsoever.
[0073]Brief Case Description.
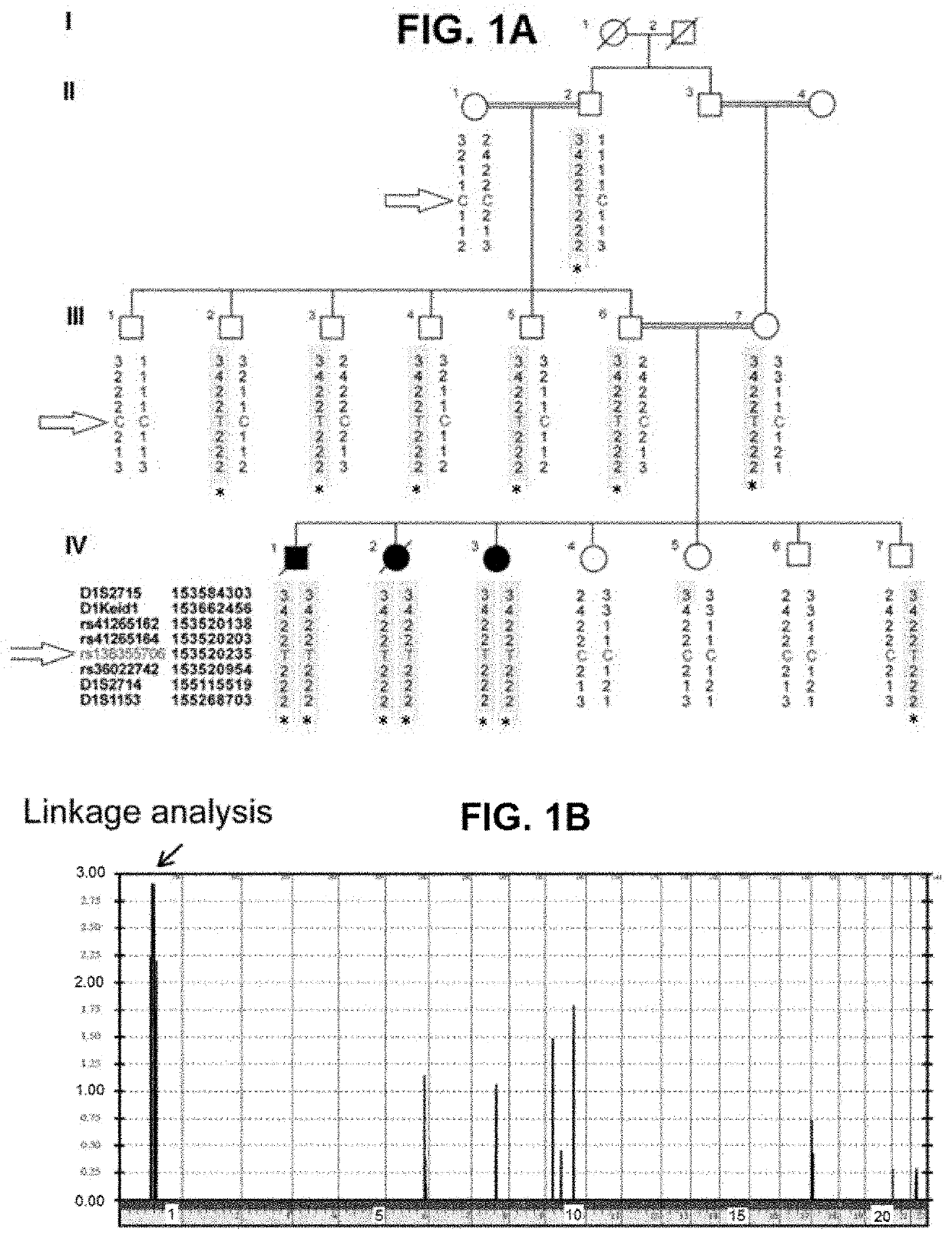
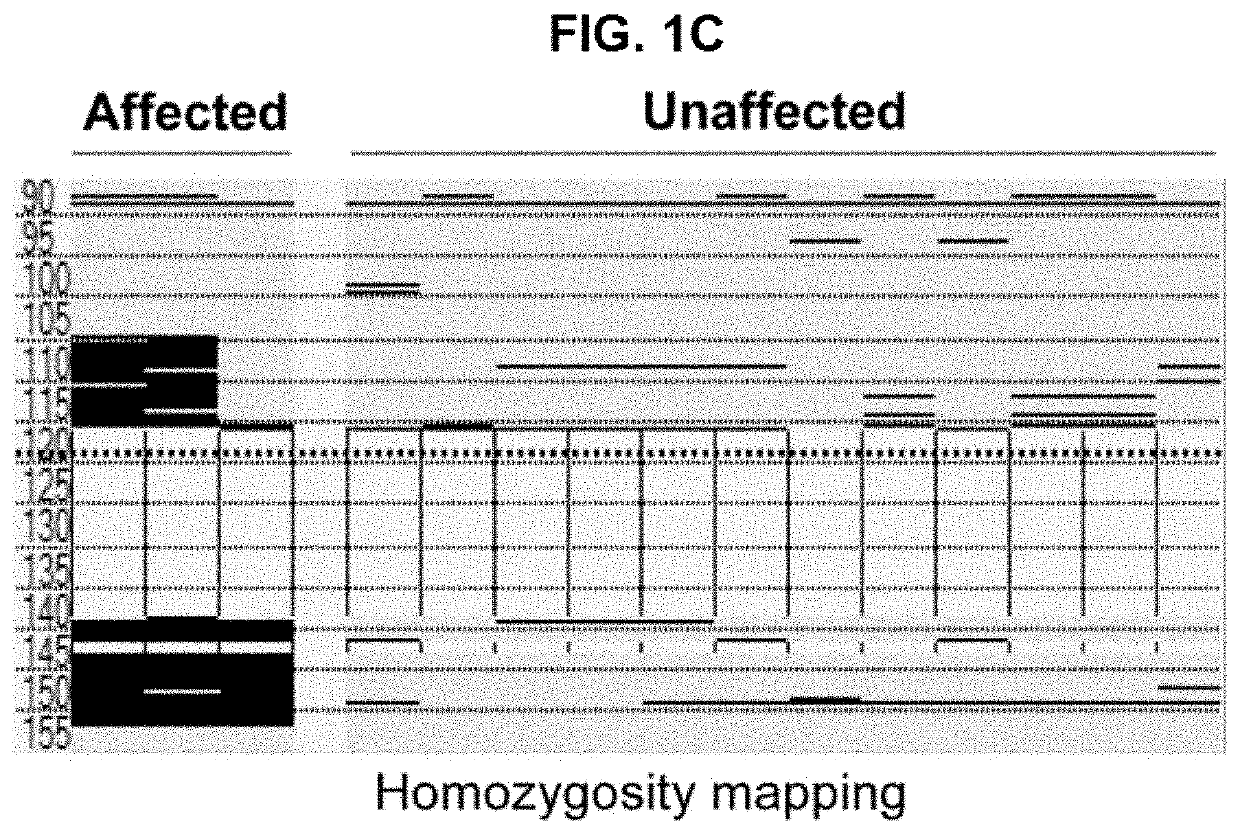
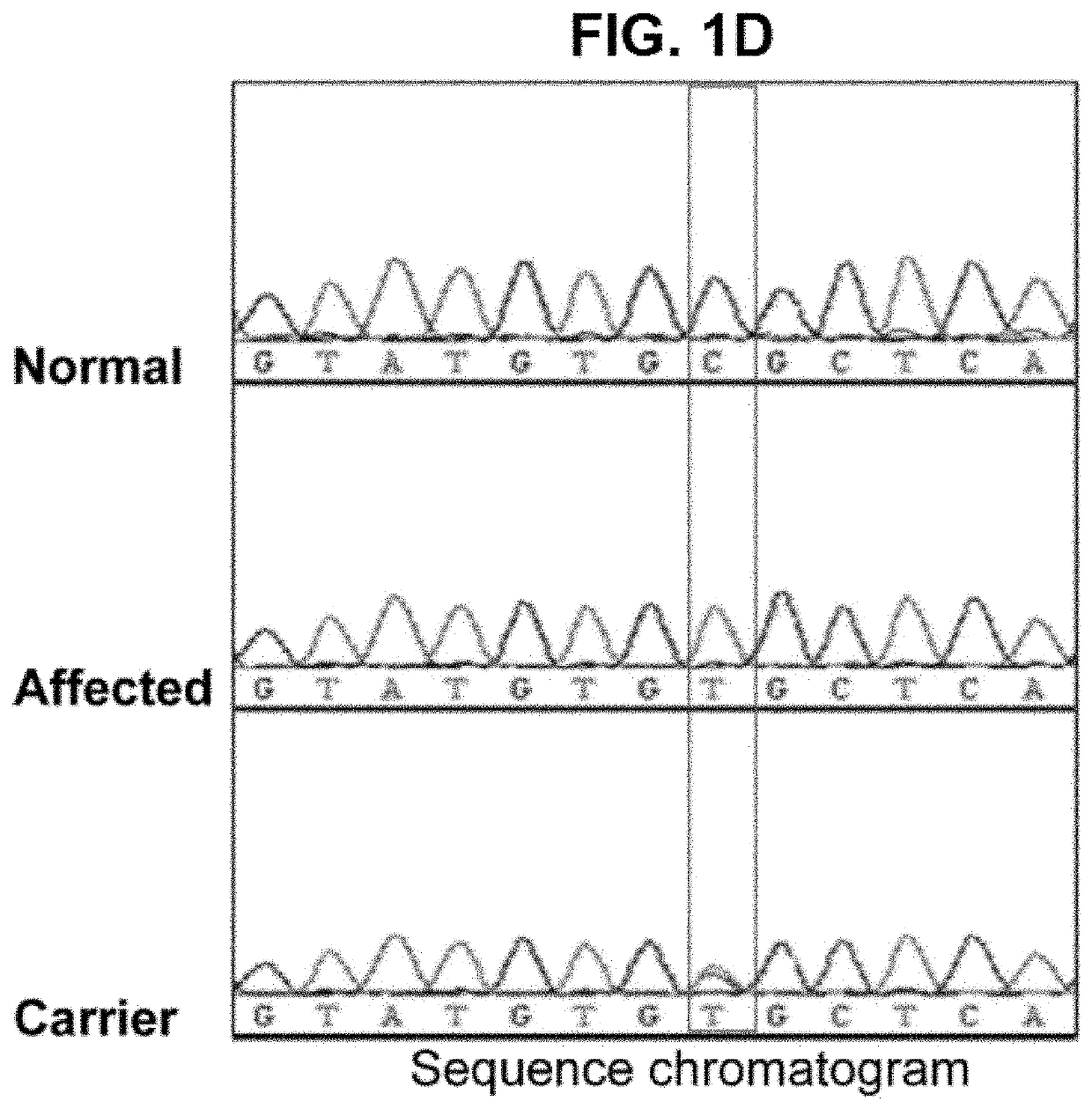
[0074]Three siblings, two girls and one boy, from a total of seven children were born healthy to consanguineous Saudi Arabian parents after normal pregnancies and deliveries (see chart in FIG. 1A). The parents did not report any medical problems until all three children developed respiratory fibrosis at a young age. All 3 affected siblings had an identical clinical presentation and course. They all developed dyspnea in their early teens and apart from the lung abnormalities, complete and extensive medical examinations revealed normal appearance, development and laboratory findings.
[0075]Pulmonary function testing showed severe restriction and impaired oxygen transfer (FIG. 2B) and fibrosis with a non-specific pattern was revealed by chest CT imaging (FIG. 2A). The subjects had extensive medical invest...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| ERS | aaaaa | aaaaa |
| full-length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com