Polypeptide, its derivative and its application in the preparation of drugs for preventing and treating tumors
A technology of derivatives and peptides, applied in the direction of antineoplastic drugs, drug combinations, peptides containing positioning/targeting motifs, etc., can solve the problems of complex signaling pathway regulation mechanism, slow and lack of drug development process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 The surface plasmon resonance method is used to screen peptides that bind to β-catenin protein.
[0034]Firstly, the TRIB3 segment is truncated into different peptide fragments, and the peptides are synthesized with a peptide solid-phase synthesizer. This process is carried out by Beijing Saibaisheng Gene Co., Ltd. The entire screening process was carried out in a surface plasmon resonance instrument Biacore T200. The screening method is as follows:
[0035] 1) The purified protein β-catenin (purchased from Beijing Yiqiao Shenzhou Co., Ltd.) was coupled to the CM5 chip (purchased from GE Company) through amino groups, and the unbound protein was washed away at a flow rate of 10 μL / min, and the surface of the chip was equilibrated 2 Hours, the buffer for washing and equilibration was glycine buffer at pH 2.5.
[0036] 2) 200 μL of different concentrations of polypeptide fragments (200, 100, 50, 25, 12.5 nM) were automatically injected, and the whole process w...
Embodiment 2
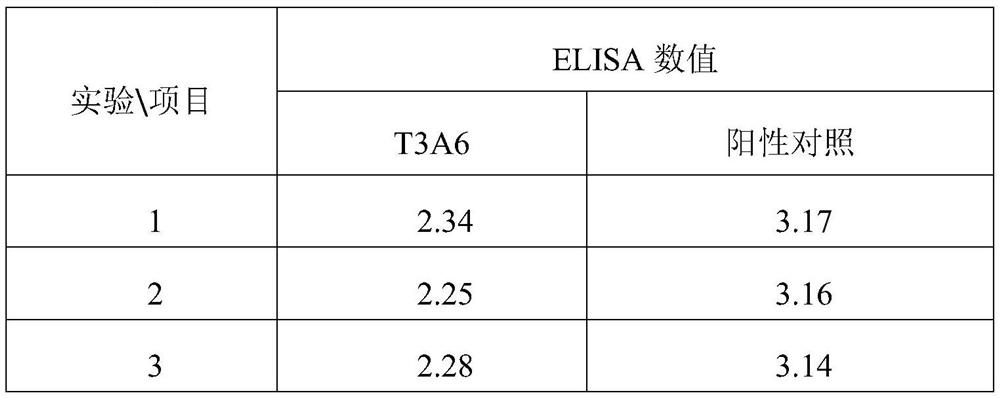
[0040] Example 2 ELISA method to verify the binding of peptide T3A6 to protein β-catenin
[0041] The specific operation steps are as follows:
[0042] 1. Dilute human β-catenin protein and bovine serum albumin (BSA) with PBS to 10 μg / ml respectively, add 100 μl to each well, and coat 96-well ELISA plate overnight at 4°C.
[0043] 2. Wash three times with PBS containing 0.1% Tween-20, coat the plate with 200 μl blocking solution (10% bovine serum PBS), and coat at 37° C. for 2 hours.
[0044] 3. Pour off the blocking solution used for coating, and add 200 μl of 1 μg / ml polypeptide T3A6 solution; at the same time, set up a positive control, add 200 μl of 1 μg / ml TRIB3 protein solution (purchased from RD Company), and incubate at 37°C for 1 hour.
[0045] 4. Wash five times with PBS containing 0.1% Tween-20, add 100 μl anti-β-catenin monoclonal antibody diluted with blocking solution 1:4000 (v / v) to each well, and incubate at room temperature for 1 hour.
[0046] 5. Wash six t...
Embodiment 3
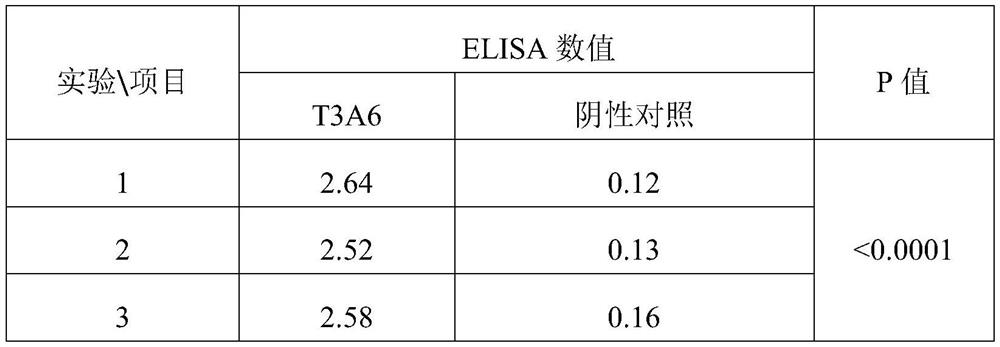
[0051] Example 3 The method of competition ELISA verifies that the peptide T3A6 can compete for the binding of TRIB3 and β-catenin protein
[0052] The specific operation steps are as follows:
[0053] 1. Dilute human β-catenin protein and bovine serum albumin (BSA) with PBS to 10 μl / ml, add 100 μl to each well, and coat 96-well ELISA plate overnight at 4°C.
[0054] 2. Wash three times with PBS containing 0.1% Tween-20, coat the plate with 200 μl blocking solution (10% bovine serum PBS), and coat at 37° C. for 2 hours.
[0055] 3. Pour off the coated blocking solution, add 200 μl of 1 μg / ml TRIB3 protein solution, and incubate at 37°C for 1 hour.
[0056] 4. Wash five times with PBS containing 0.1% Tween-20; add 100 μl of blocking solution to each well as a negative control and 100 μl of horseradish catalase-labeled polypeptide T3A6 diluted with blocking solution, and incubate at room temperature for 1 h.
[0057] 5. Wash six times with PBS containing 0.1% Tween-20; prepare...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com