Processes for preparing 4-methyl-5-nonanone and 4-methyl-5-nonanol
a technology of 4methyl-5-nonanone and process, which is applied in the preparation of carbonyl compounds, carboxylic compound preparations, organic chemistry, etc., can solve the problems of difficult industrial production of reactors, and achieve the effects of low cost, high yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
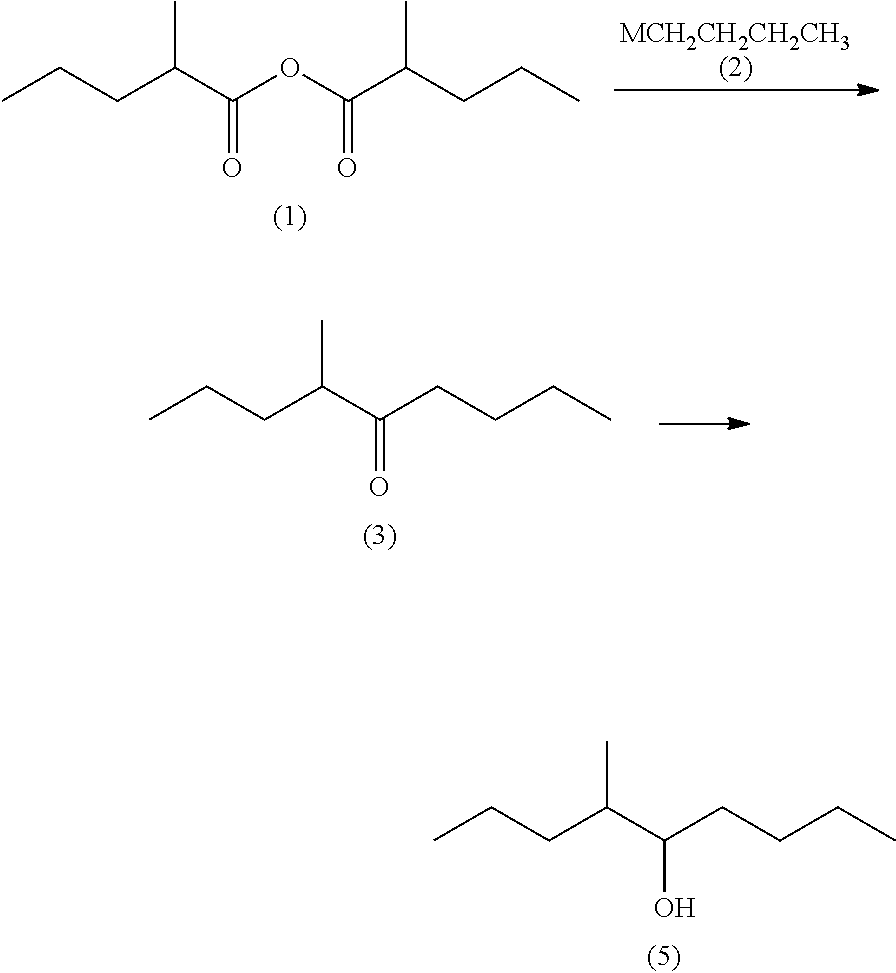
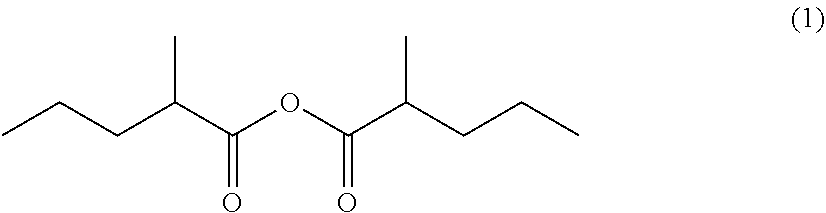
on of 2-methylpentanoic anhydride (1)
[0112]
[0113]First, a distillation tower was corrected to one of the ports of a reactor and a fractionating tower was connected to the outlet of the, distillation tower. Further, a thermometer and a condenser were connected to the fractionating tower.
[0114]The 2-methylpentanoic acid (4) (459.41 g, 3.955 mol) and acetic anhydride (Ac2O) (817.33 g, 7.91 viol) were added to the aforesaid reactor at room temperature. The fractionating tower was then closed and the mixture was stirred at an internal temperature of 160° C. and normal pressure for 30 minutes. Next, the fractionating tower was opened and acetic acid was subjected to distillation at an internal temperature of 160° C. and normal pressure, until an acetic acid content in the distillate containing at least 2.0 acetic acid, acetic anhydride, and acetic 2-methylpentanoic anhydride became 5.0%. Further, the pressure was reduced gradually to 64 mmHg at an internal temperature of 160° C. to distil...
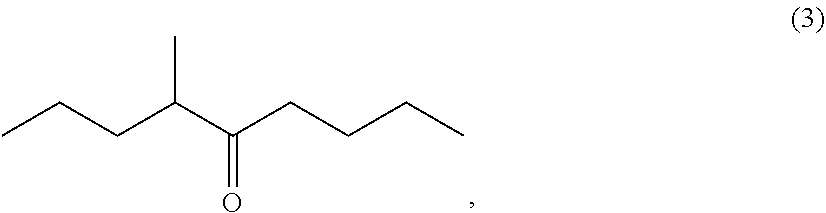
example 2a
n of 4-methyl-5-nonatione (3)
[0116]
[0117]Magnesium (54.42 g, 2.27 gram atoms) and tetrahydrofuran (THF) (639.90 g) were added to a reactor at room temperature and stirred at from 60 to 65° C. for 30 minutes. After the stirring, 1-chlorobutane (197.17 g, 2.13 mol) was added dropwise to the reactor at from 60 to 75° C. and the reaction mixture was stirred at from 75 to 80° C. for 2 hours to prepare butylmagnesium chloride (2: M═Cl).
[0118]Then, tetrahydrofuran (792.26 g) and 2-methylpentanoic anhydride (1) (456.46 g, 2.13 mol) were added to another reactor and the whole amount of the butylmagnesium chloride (2: M═Cl) obtained above was added dropwise at from −5 to 10° C. After the completion of the dropwise addition, the reaction mixture was stirred at from 0 to 10° C. for 3 hours, Then, acetic acid (168.05 g) and water (826.68 g) were added to the reaction mixture in the reactor to cause phase separation and the aqueous phase thus obtained was removed. An aqueous 25 wt % sodium hydrox...
example 2b
f 2-methyl-1-pentanoic Acid (4)
[0120]
[0121]The aqueous phase (2604.29 g) containing sodium 2-methylpentanoate (7: Y═Na) obtained in Example 2A was added to a reactor and an aqueous 20 wt % hydrochloric acid (570.93 g, 3.13 mol of hydrogen chloride) was added dropwise at from 10 to 20° C. After the completion of the dropwise addition, the reaction mixture was stirred at from 15 to 25° C. for one hour. The reaction mixture was left to stand. After 2-methyl-1-pentanoic acid (4) was liberated and the reaction mixture separated into an organic phase and an aqueous phase, the aqueous phase was removed by a phase separation to take up the Organic phase containing the 2-methyl-1-pentatonic acid (4). It was confirmed by a 071 test strip that the aqueous phase had a pH of 1.0. The organic phase thus obtained was concentrated at a reduced. pressure to obtain 2-methyl-1-pentanoic acid (4) (179.19 g, 2.03 mol) in a yield of 95.3%.
[0122]The following is spectrum data of the 2-methyl-1-pentanoic a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight ratio | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com