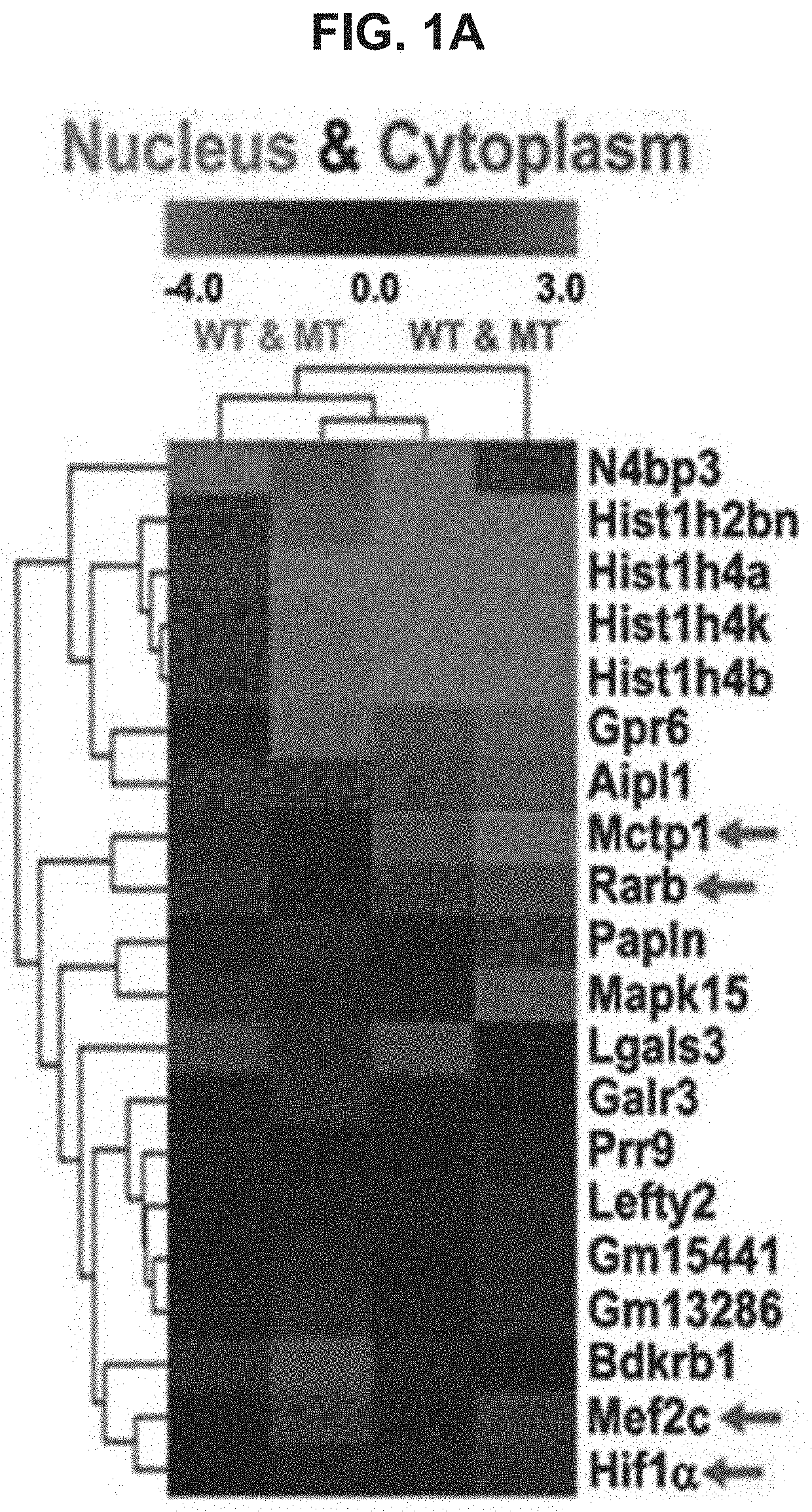
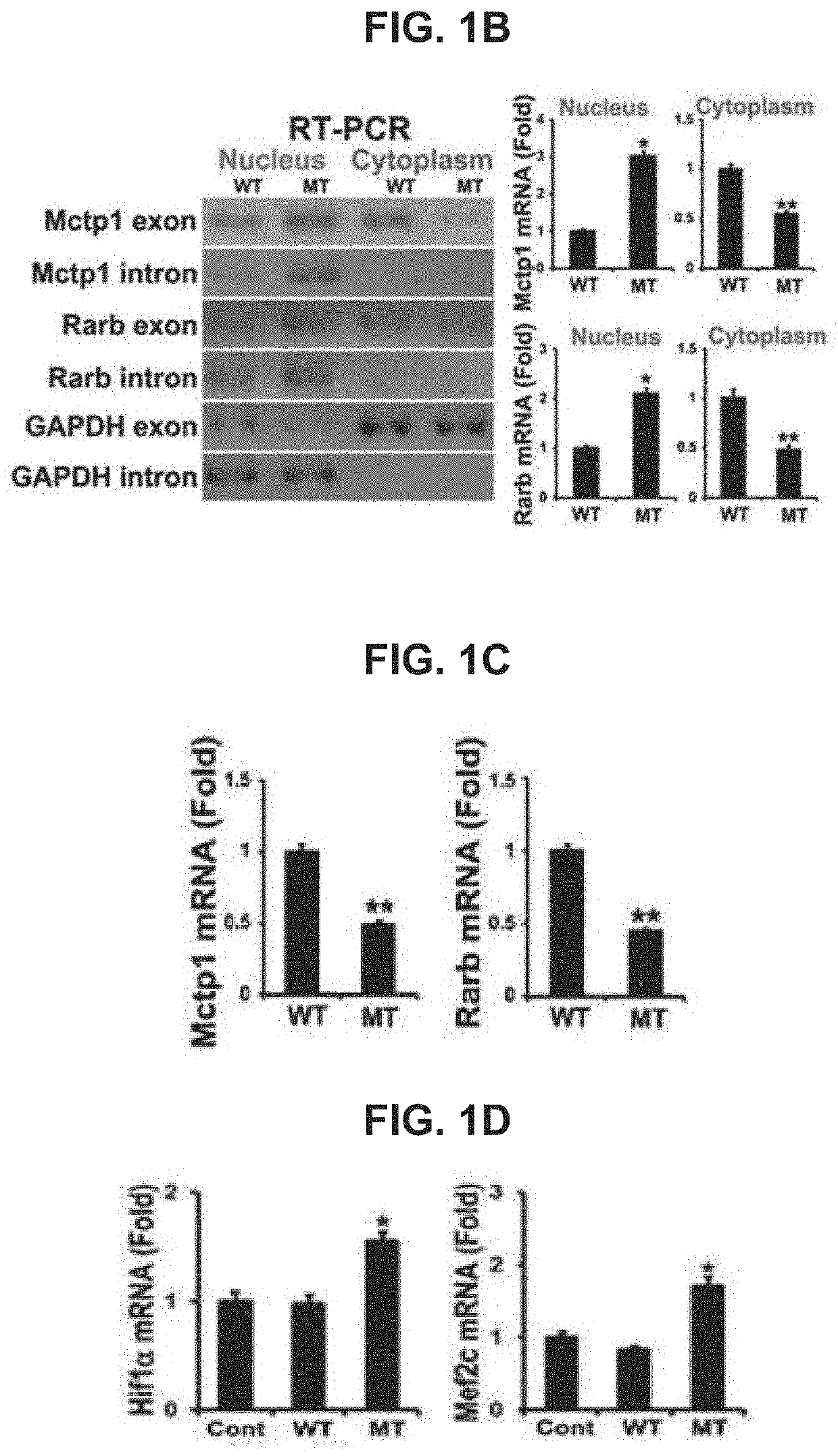
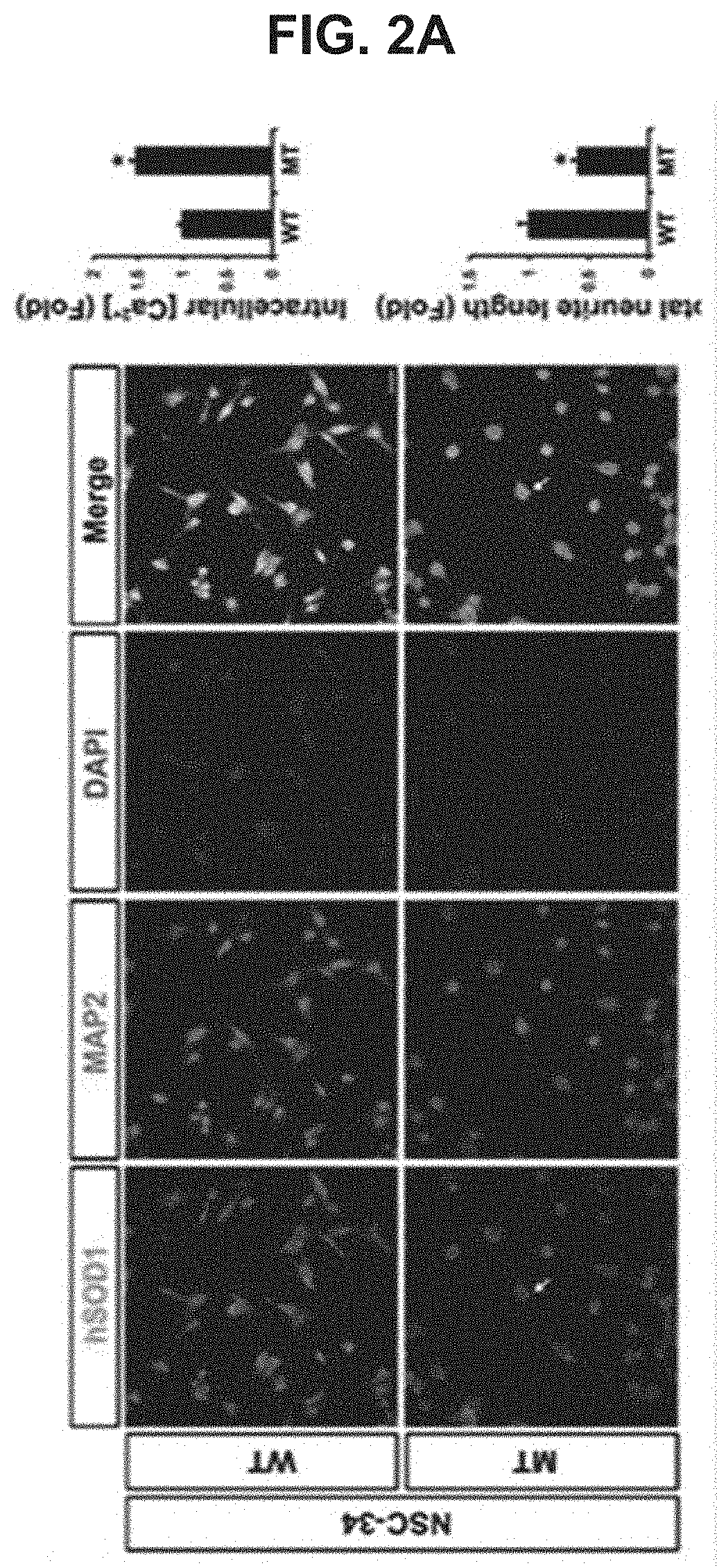
USE OF miR-18b FOR PREVENTION, TREATMENT, OR DIAGNOSIS OF MUSCLE DISEASE AND NEUROMUSCULAR DISEASE
a neuromuscular disease and mir-18b technology, applied in the direction of muscular disorders, drug compositions, genetic material ingredients, etc., can solve the problems of few options for slowing down disease progression or improving the quality of life of als patients, hypotonia, myoatrophy, etc., to reduce mir-18b expression, recover calcium signaling and cell differentiation, and suppress apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0044]Hereinafter, the present invention is described in more detail.
[0045]The present invention provides a method for preventing or treating muscle diseases, including administering a composition comprising a pharmaceutically effective amount of miR-18b to a subject.
[0046]According to the present invention, miR-18b may be derived from animals including humans, such as monkeys, chimpanzees, pigs, horses, cows, sheep, dogs, cats, mice, rabbits.
[0047]According to the present invention, the nucleic acid molecule forming miR-18b may have a length of 18 to 100 nt (nucleotide). Specifically, the nucleic acid molecule may be in the form of a mature miRNA having a length of 19 to 25 nt, more specifically having a length of 21, 22, or 23 nt. In addition, the nucleic acid molecule may be in the form of a precursor miRNA having a length of 50 to 100 nt, more specifically having a length of 65 to 95 nt.
[0048]In addition, the miR-18b in the form of mature miRNA may specifically be miR-18b-5p or ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com