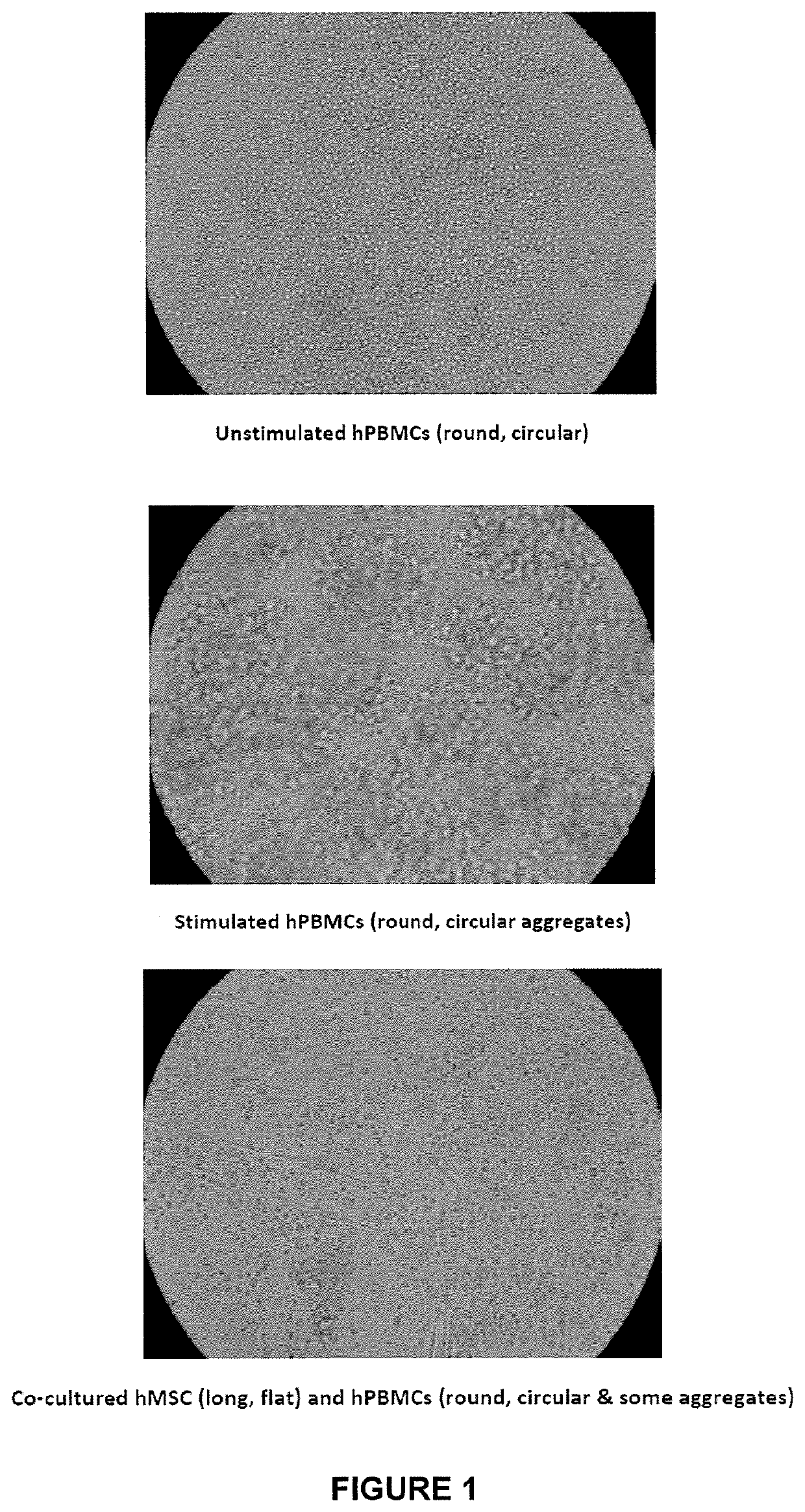
Mesenchymal lineage precursor or stem cells with enhanced immunosuppression
a mesenchymal lineage and immunosuppression technology, applied in the direction of drug compositions, skeletal/connective tissue cells, biochemistry apparatus and processes, etc., can solve the problems of inability to confirm the biological activity and potency of a product by physiochemical parameters, and difficult to define consistent cellular therapy product quality, etc., to achieve the effect of improving immunosuppressive activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
Mesenchymal Lineage Precursor or Stem Cells (MLPSCs) Prepared Using Plastic Adherence Techniques
[0353]MLPSCs were generated de novo from bone marrow as described in U.S. Pat. No. 5,837,539. Approximately 80-100 ml of bone marrow was aspirated into sterile heparin-containing syringes and taken to the MDACC Cell Therapy Laboratory for MSC generation. The bone marrow mononuclear cells were isolated using ficoll-hypaque and placed into two T175 flask with 50 ml per flask of MLPSC expansion medium which includes alpha modified MEM (αMEM) containing gentamycin, glutamine (2 mM) and 20% (v / v) fetal bovine serum (FBS) (Hyclone).
The cells were cultured for 2-3 days in 37° C., 5% CO2 at which time the non-adherent cells were removed; the remaining adherent cells were continually cultured until the cell confluence reached 70% or higher (7-10 days), and then the cells were trypsinized and replaced in six T175 flasks with expansion medium (50 ml of medium per flask).
Immunose...
example 2
ected Mesenchymal Lineage Precursor or Stem Cells with Enhanced Immunosuppressive Properties
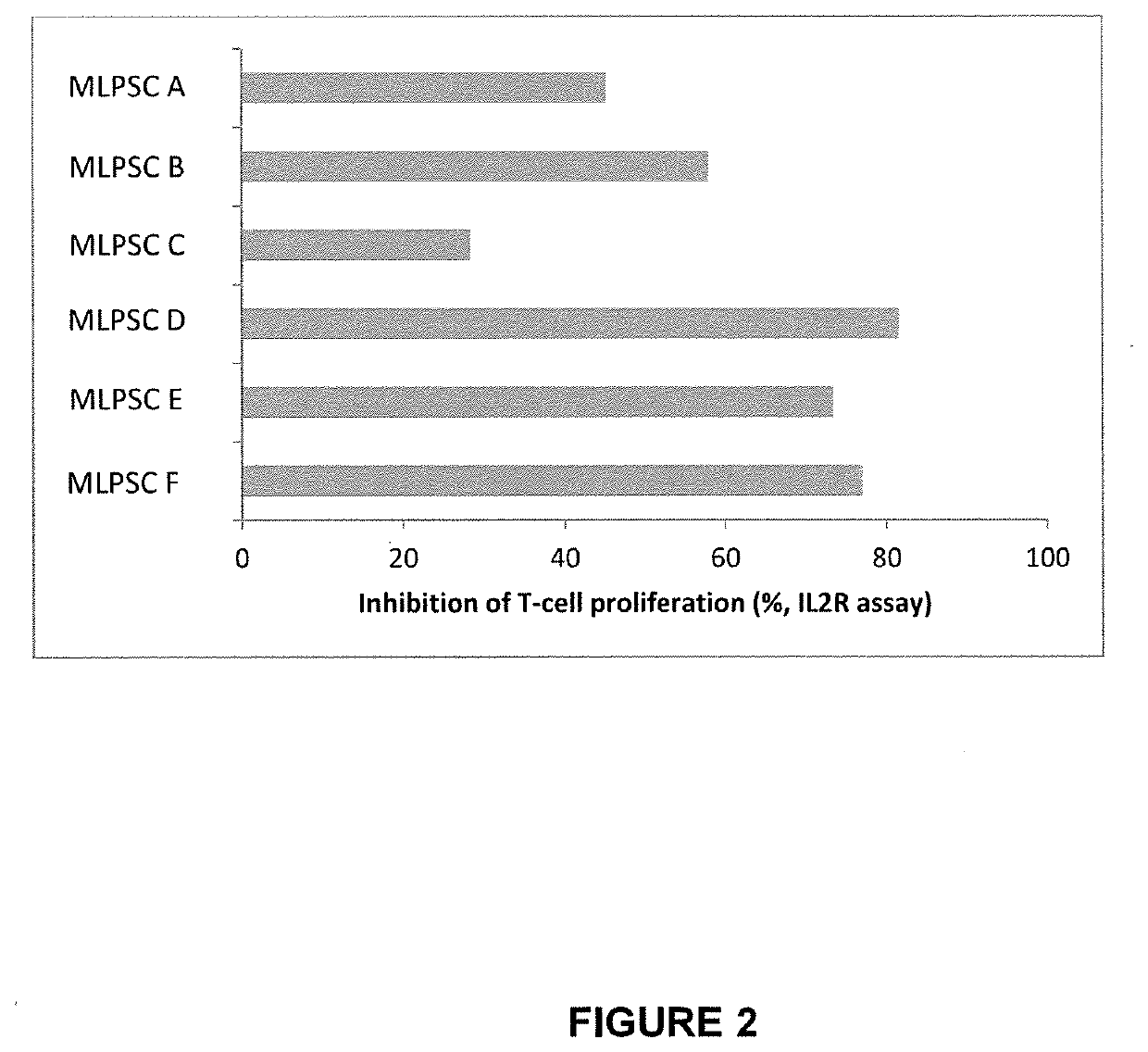
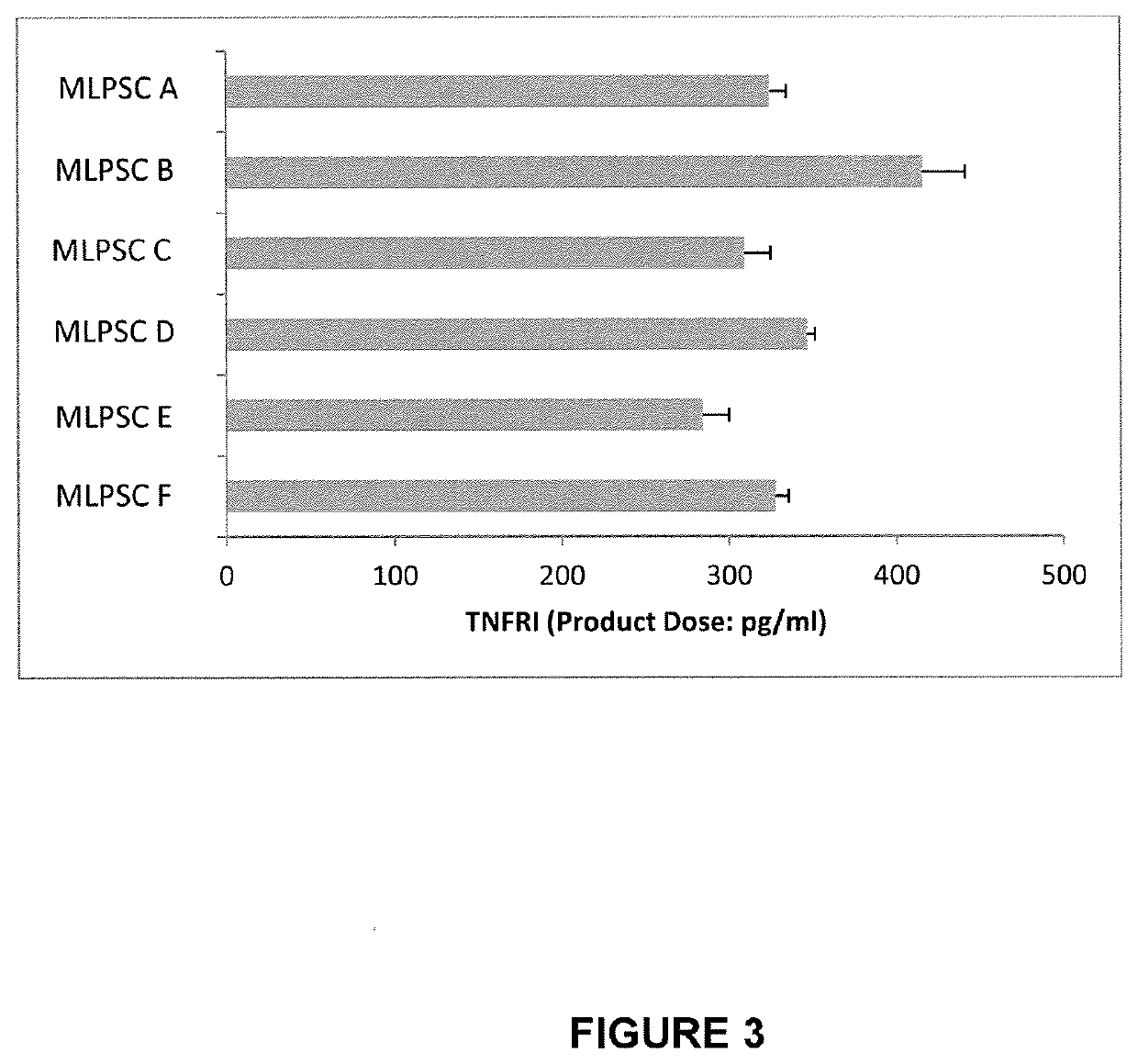
[0386]The immunosuppressive potential of immunoselected mesenchymal lineage precursor or stem cells (MLPSCs) were assess by comparison with products obtained by the previous manufacturing process (as described in U.S. Pat. No. 9,828,586). Results of the T cell proliferation assay (% inhibition of IL2R) performed on three different samples of MLPSCs produced under the previous manufacturing conditions (i.e. samples MLPSC A, MLPSC B and MLPSC C) and three different samples of improved immunoselected MLPSCs with (i.e. samples MLPSC D, MLPSC E and MLPSC F) compared to are shown in FIG. 2. These results show that the assay differentiates between a first class of multipotential lineage cells and a second class of multipotential lineage cells in terms of ability to inhibit T-cell proliferation. In particular, the second class of cells inhibits T cell proliferation substantially more effectively that...
example 3
: Mesenchymal Lineage Precursor or Stem Cell (MLPSC) with Low IL-2R Inhibition Infused for the Treatment of Pediatric Subjects Who are Steroid Refractory
[0389]This study is for the treatment of pediatric patients who have failed to respond to steroid treatment for acute graft-versus-host disease (GVHD). Failing steroid treatment for acute GVHD is defined as any Grade B-D (IBMTR grading) of acute GVHD that is not improving after at least 3 days of methylprednisolone≥1 mg / kg / day) or equivalent.
[0390]Patients were treated with ex-vivo cultured MLPSC with low IL-2R inhibition twice per week at a dose of 2×106 hMSC / kg (actual body weight) for each of 4 consecutive weeks. Infusions were administered at least 3 days apart.
[0391]Study Design:
[0392]241 pediatric patients undergoing HSCT were enrolled and treated at 50 sites in North America and Europe from 2007-2014
[0393]Ages 2 months-17 years
[0394]Acute GvHD grades B-D (CIBMTR)
[0395]Failed steroid treatment and multiple other agents
[0396]aG...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com