Methods of treating depression with vortioxetine
a technology of vortioxetine and depression, which is applied in the direction of nervous disorders, medical preparations, drug compositions, etc., can solve the problems of increasing the patient's risk of serotonin syndrome, life-threatening or fatal, and the perception of the risk of transitioning from treatment with vortioxetine to maoi
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
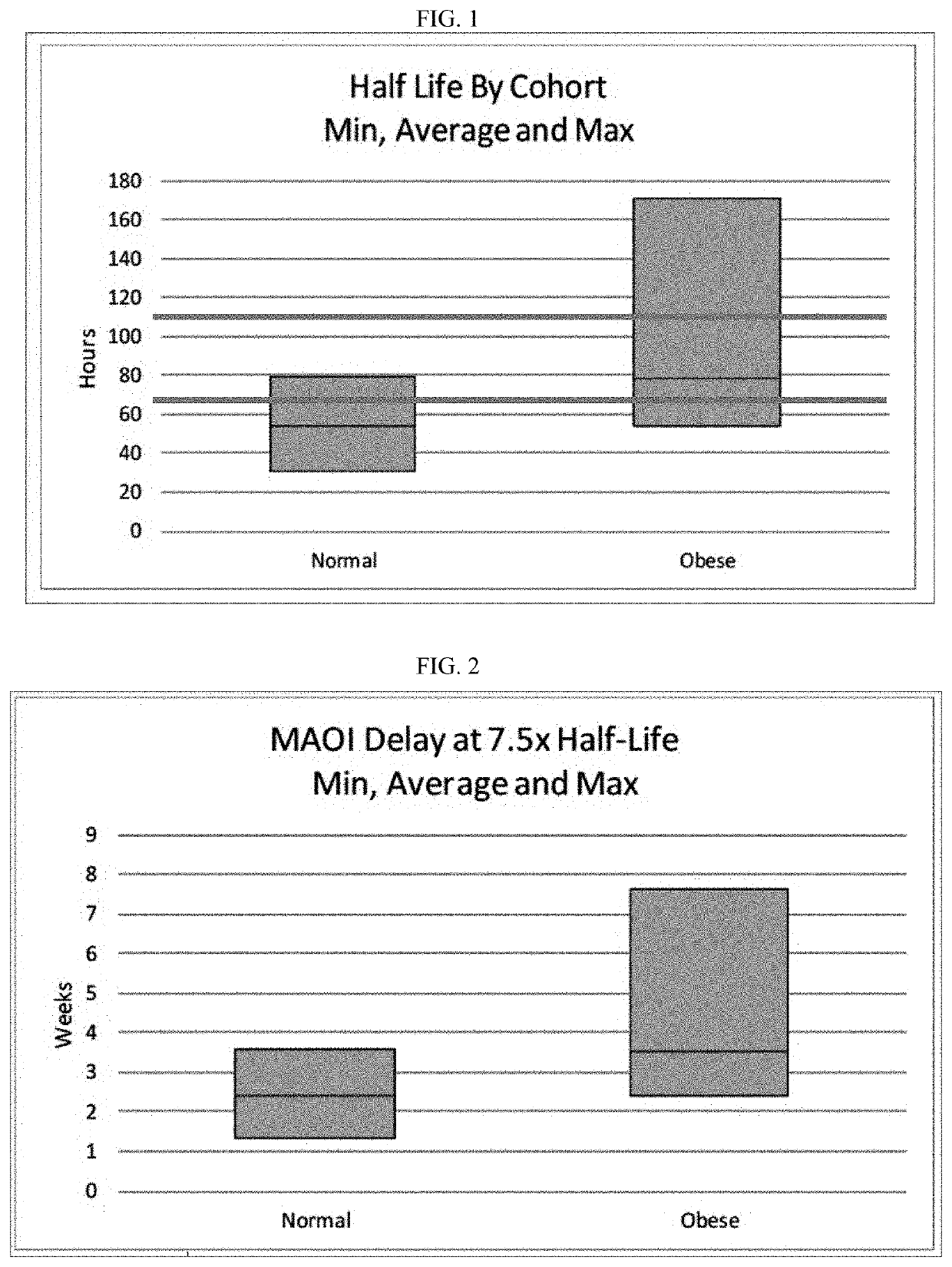
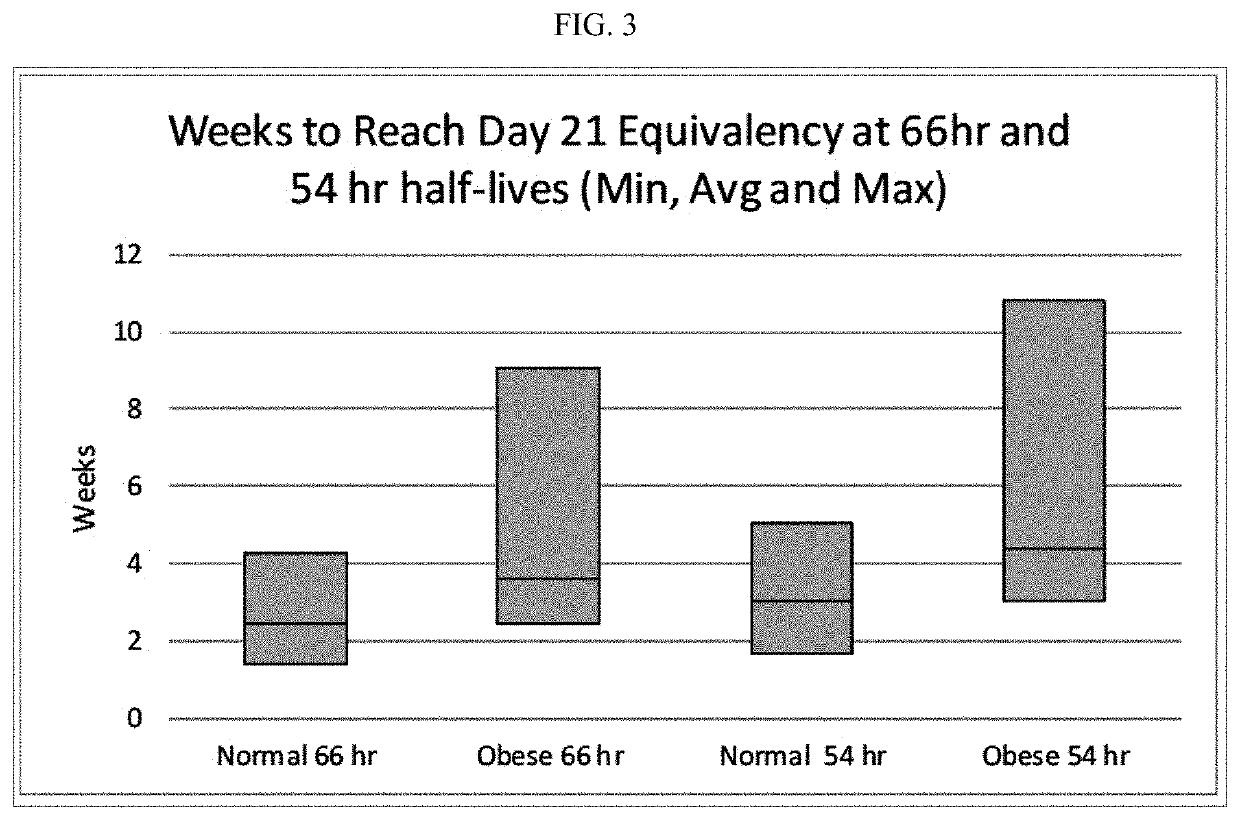
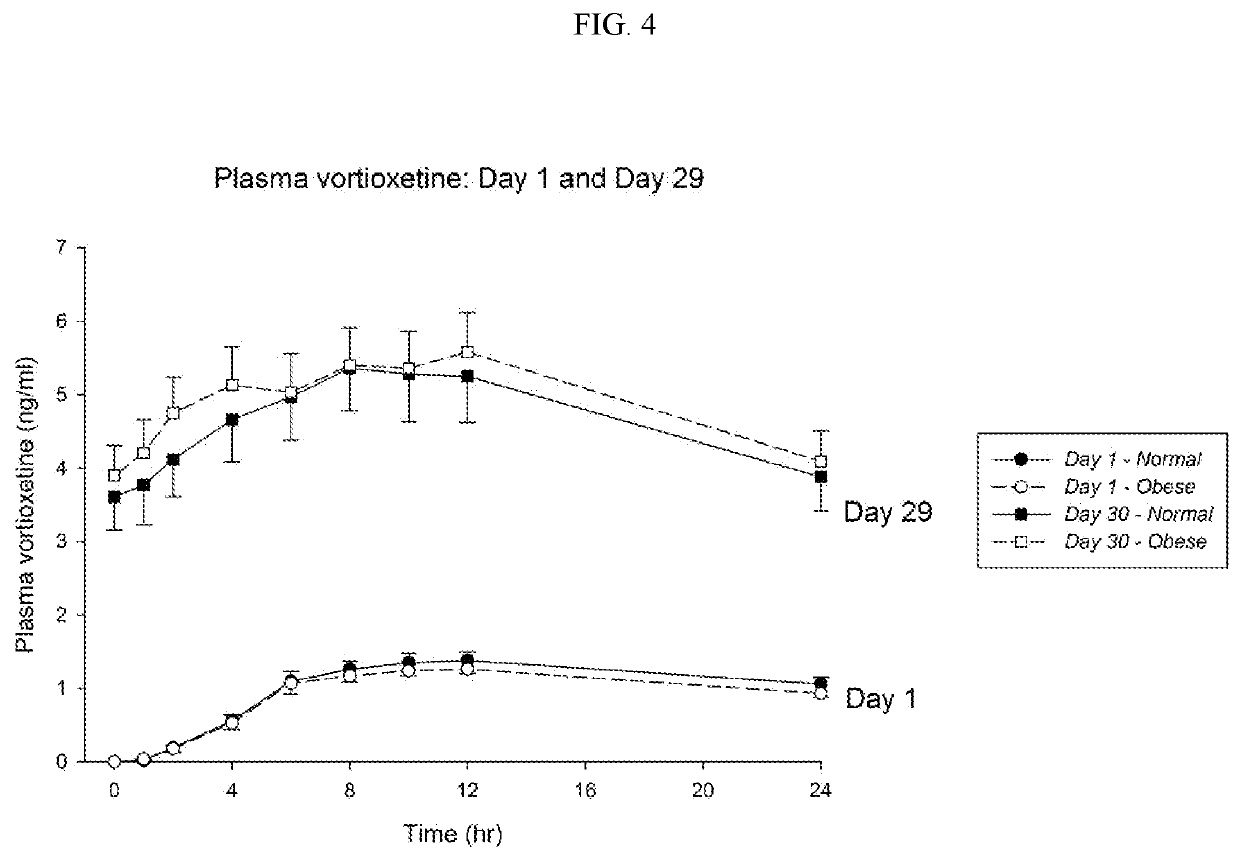
[0157]To test if the obese patients would require different washout guidelines for preventing serotonin syndrome, 16 obese subjects (BMI≥35) and 13 normal subjects (BMI<25) completed a clinical trial where they were administered daily with low doses (5 mg / day) of vortioxetine for 29 days in a controlled study. All subjects were subsequently tracked for 28 days after discontinuing vortioxetine on day 29. Blood samples were collected and processed according to standard techniques.
[0158]The weight, BMI, waist measurement, % body weight, % total fat, % android fat, % gynoid fat, total fat, metabolizer status, and measured half-life of each subject in shown in Table 1, below.
TABLE 1Vortioxetine Half-Life and Subject CharacteristicsCss%%%TotalT1 / 2(ng / WeightBMIWaist%TotalAndroidGynoidFatMetab.Subject(h)mL)(kg)(kg / m2)(in)IBWFatFatFat(kg)StatusCohort01_00863.95.4263.022.630.7107.738.238.140.524.1EMNormal01_00952.84.1462.721.731.5102.339.140.442.924.5EMNormal01_01050.73.1362.223.631.5105.428....
example 2
[0164]Data obtained in the study described in Example 1 and Table 1 provided the basis for a regression model (having a r2 value of 88%) based on total body fat and steady-state vortioxetine plasma concentration. Because the steady-state plasma concentration of vortioxetine correlates with metabolizer status, this regression provides a means of estimating vortioxetine half-life for patients based on total body fat and CYP2D6 metabolizer status. The results are shown in Table 4.
TABLE 4Half-Life Estimates (hrs) Based on Total Body Fat (kg) and Metabolizer StatusTotal Fat (Kg)102030405060708090100Meta-Extensive(a)2132425262738393103114bolizerExtensive(b)394959708090100111121131StatusIM(c)102112123133143153164174184194IM(d)116126136147157167177188198208PM(e)215225235245256266276286297307(a)average of obese steady-state concentration for extensive metabolizers(b)high of extensive metabolizers(c)average of intermediate metabolizer (IM) values(d)high of IM values(e)estimated poor metaboliz...
example 3
[0167]The clinical trials in Examples 1 and 2 are discussed in more detail below.
Overall Design
[0168]The normal-weight control group consisted of healthy male and female volunteers aged 18 to 45 years. Their body mass index (BMI) values fell in the range of 18.5 to 25 kg / m2. The overweight subjects had a BMI of at least 35 kg / m′. Normal weight and obese subjects were matched as closely as possible for gender and age.
[0169]Potential study participants underwent screening and evaluation within 30 days of study initiation. Procedures included: medical and psychiatric history, physical examination, electrocardiogram if indicated, hematologic and biochemical screening, and urine testing for drugs of abuse. All study participants were healthy active non-smoking adults with no history of significant medical or psychiatric disease, and taking no prescription medications. Overweight subjects were free of metabolic or other complications of obesity. Potentially child-bearing women in both gro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| waist size | aaaaa | aaaaa |
| waist size | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com