Uses of myostatin antagonists, combinations containing them and uses thereof
a technology of myostatin and activin, which is applied in the field of activin type ii (actrii) receptor inhibitors, can solve the problems of cisplatin precipitating body and muscle weight loss, and achieve the effect of preventing the formation of apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Bimagrumab Prevents Cisplatin-Induced Body Weight Loss
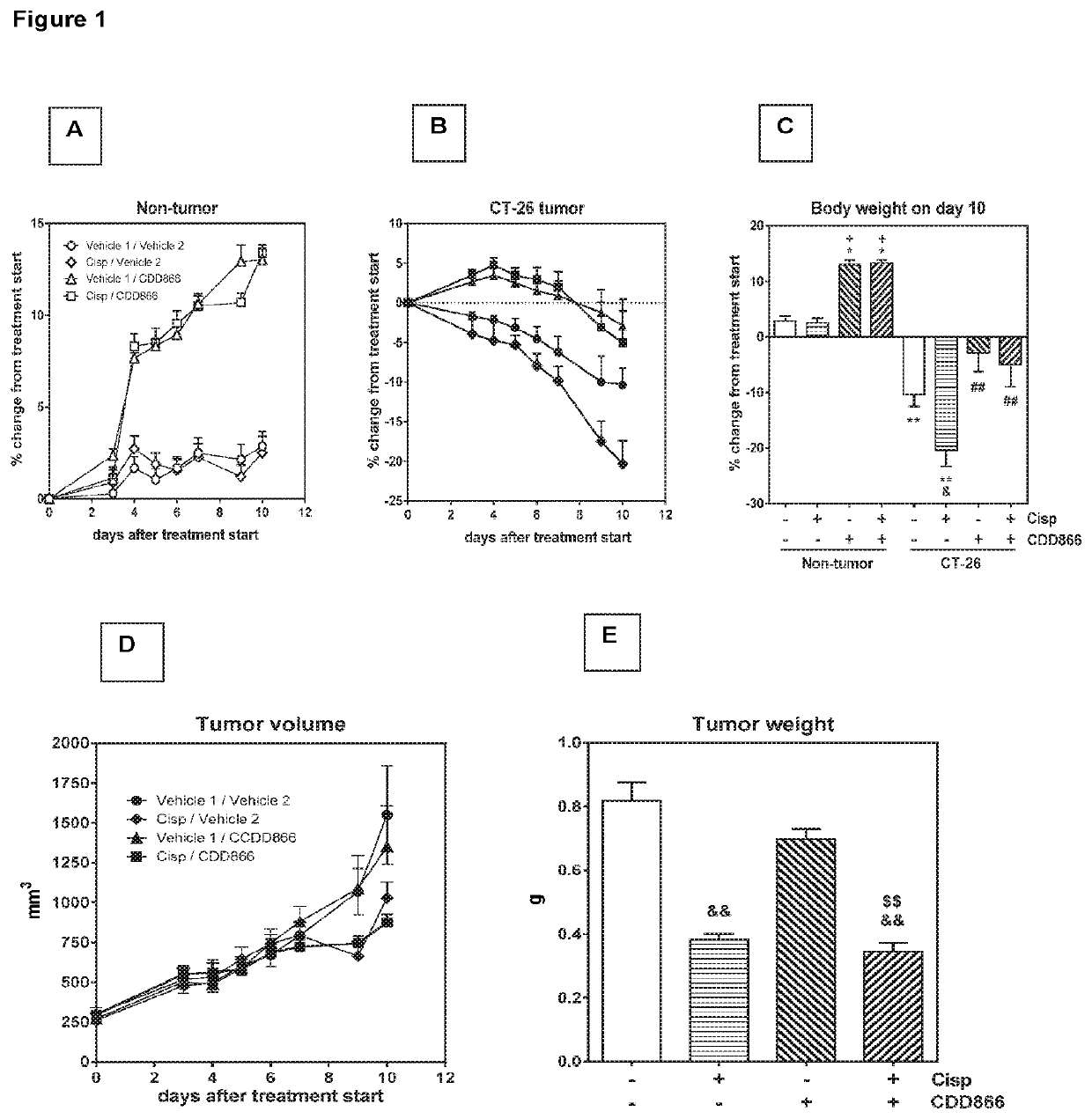
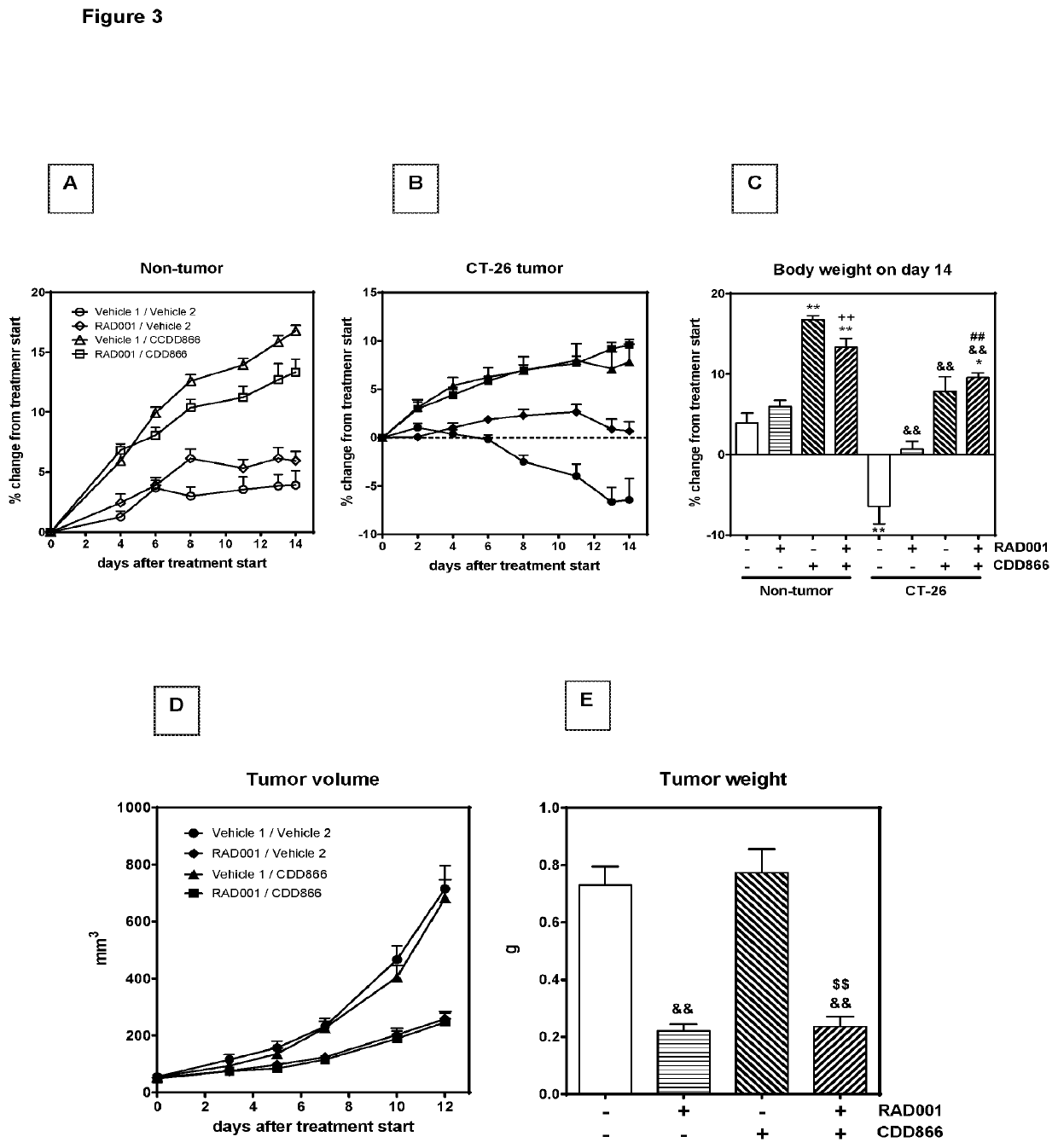
[0163]Extensive body weight loss has emerged as a key determinant of cancer-related death. Thus longitudinally body weight development was monitored (FIGS. 1A and B). Ten days after starting the treatment, tumor-bearing animals receiving cisplatin as a mono-therapy had lost 20% of their initial body weight (FIGS. 1B and C). By contrast, vehicle-treated, tumor-bearing animals experienced a body weight decrease of 10%, while animals treated with CDD866 alone or in combination with cisplatin exhibited moderate body weight losses of only 3 and 5%, respectively (FIGS. 1B and C). In healthy control animals, cisplatin did not affect body weight and CDD866 administration resulted in a marked body weight gain in the absence and presence of cisplatin (FIGS. 1A and C). These data demonstrate that cisplatin, at an effective anti-tumor dose (cf. FIG. 1E), indeed precipitated body weight loss in cachectic animals and that CDD866 significantly ...
example 2
Bimagrumab Antagonizes Cisplatin-Induced Muscle Wasting
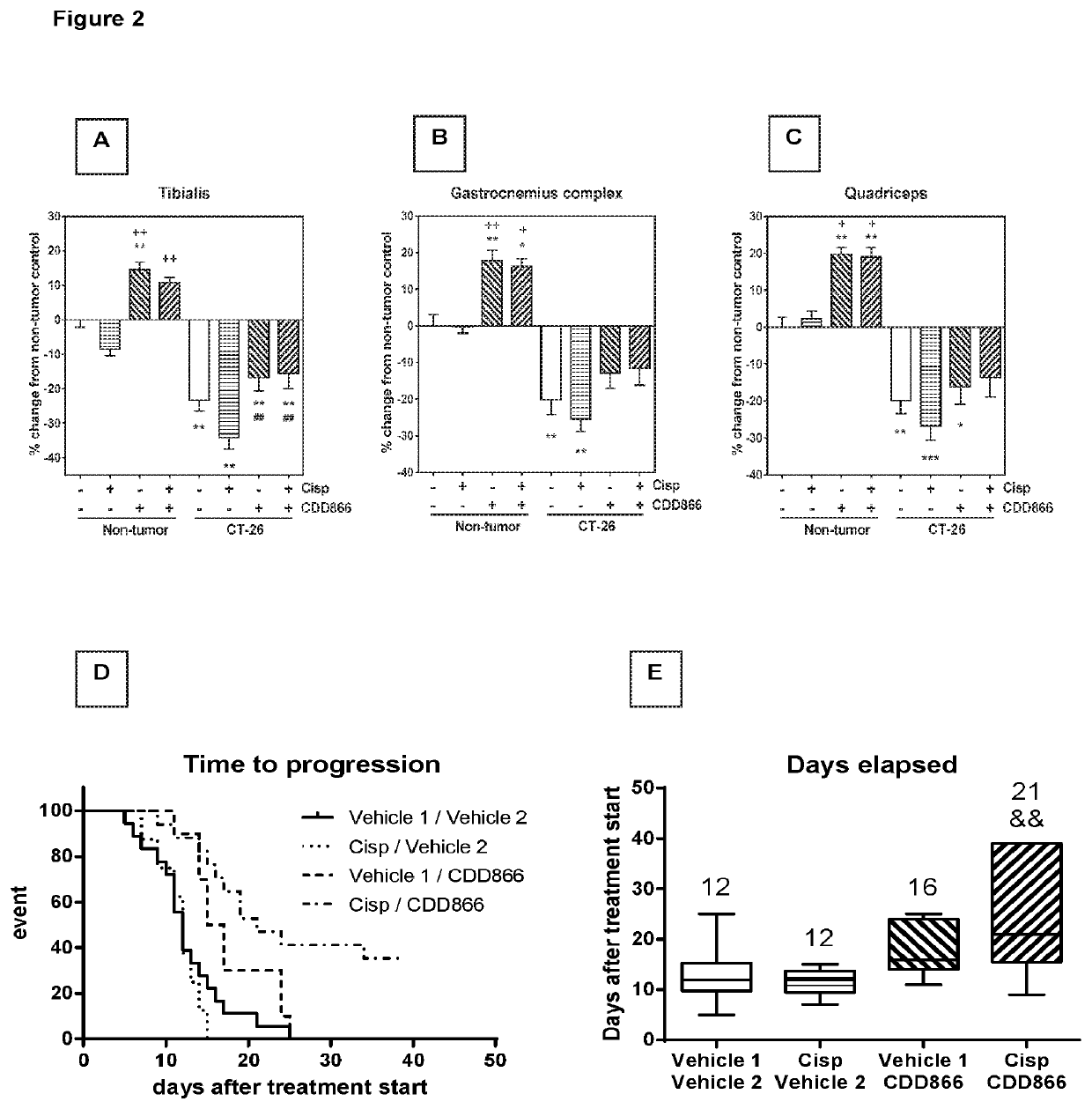
[0165]Given the positive effect of CDD866 on body weight, we next determined the impact of the various interventions on individual skeletal muscles. In gastrocnemius, cisplatin provoked a muscle weight loss of 25%. CDD866 treatment tended to reduce muscle weight loss to 13% and this protective effect was preserved in the presence of cisplatin (12%) (FIG. 2B). A similar level of protection was observed in quadriceps muscle (FIG. 2C). Tibialis anterior benefited most from CDD866 treatment. In tibialis anterior, cisplatin-treated animals experienced a muscle wasting of 34% and co-administration of CDD866 reduced muscle loss significantly to 16% (FIG. 2A).
example 3
Bimagrumab in Combination with Cisplatin Delays Time to Progression in Cancer Cachexia
[0166]Extensive tumor growth and subsequent body weight loss are important predictors of mortality in cancer patients. We therefore wanted to evaluate whether the combination of CDD866 and cisplatin has an impact on the length of survival. For ethical reason we abstained from classical survival studies. Instead, each mouse was individually euthanized when experiencing either a body weight loss exceeding 20% of initial body weight, or reaching a tumor volume of 1,500 mm3, determined as time-to-progression.
[0167]On average, animals receiving vehicle or cisplatin had to be sacrificed after 12 and 12 days, respectively (FIGS. 2D and E). CDD866 treated animals had to be euthanized after 16 days, which corroborates previous findings that CDD866 treatment reduced body weight loss, but did not promote tumor growth. The combined treatment of CDD866 and cisplatin was superior to any other intervention tested...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com