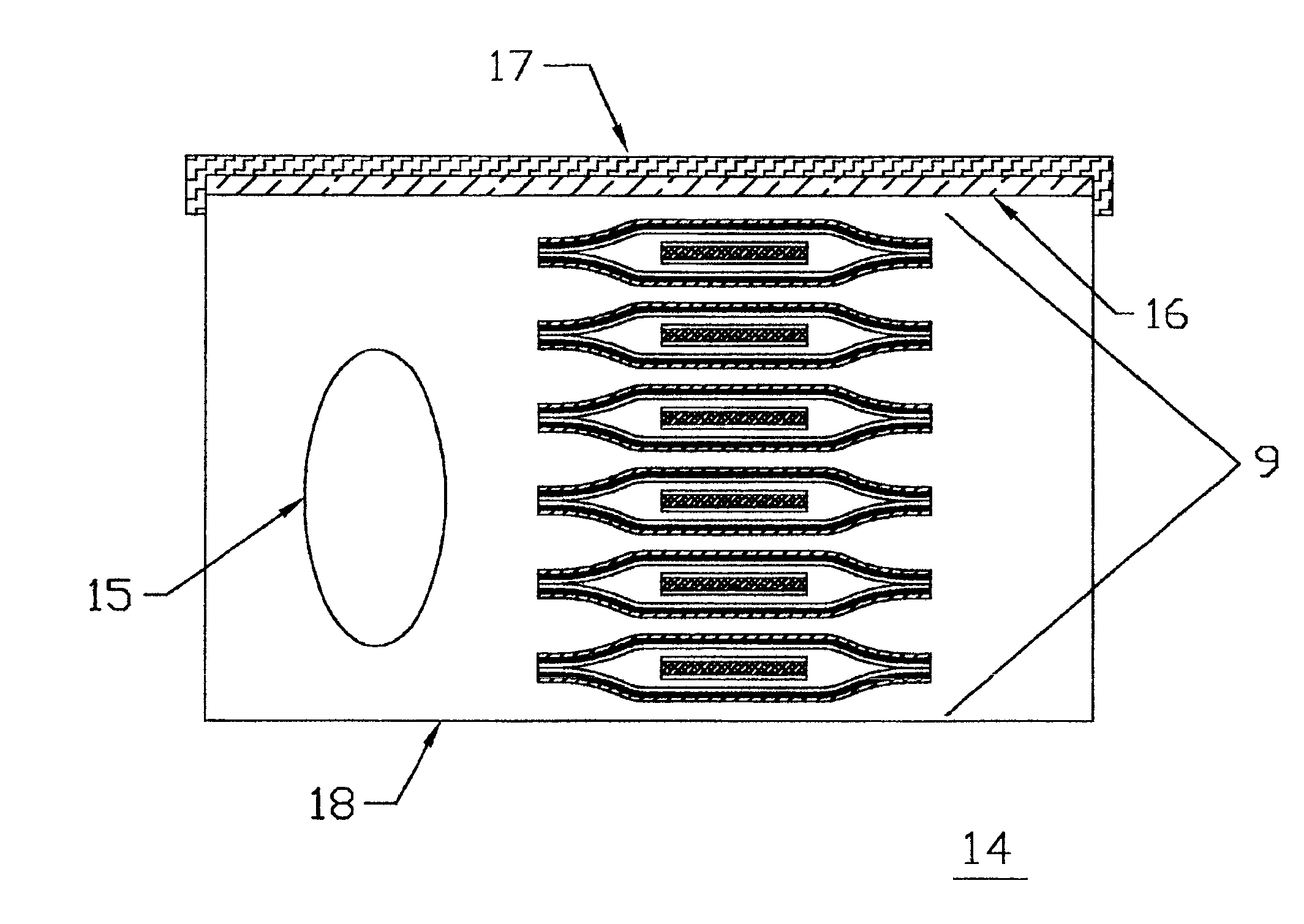
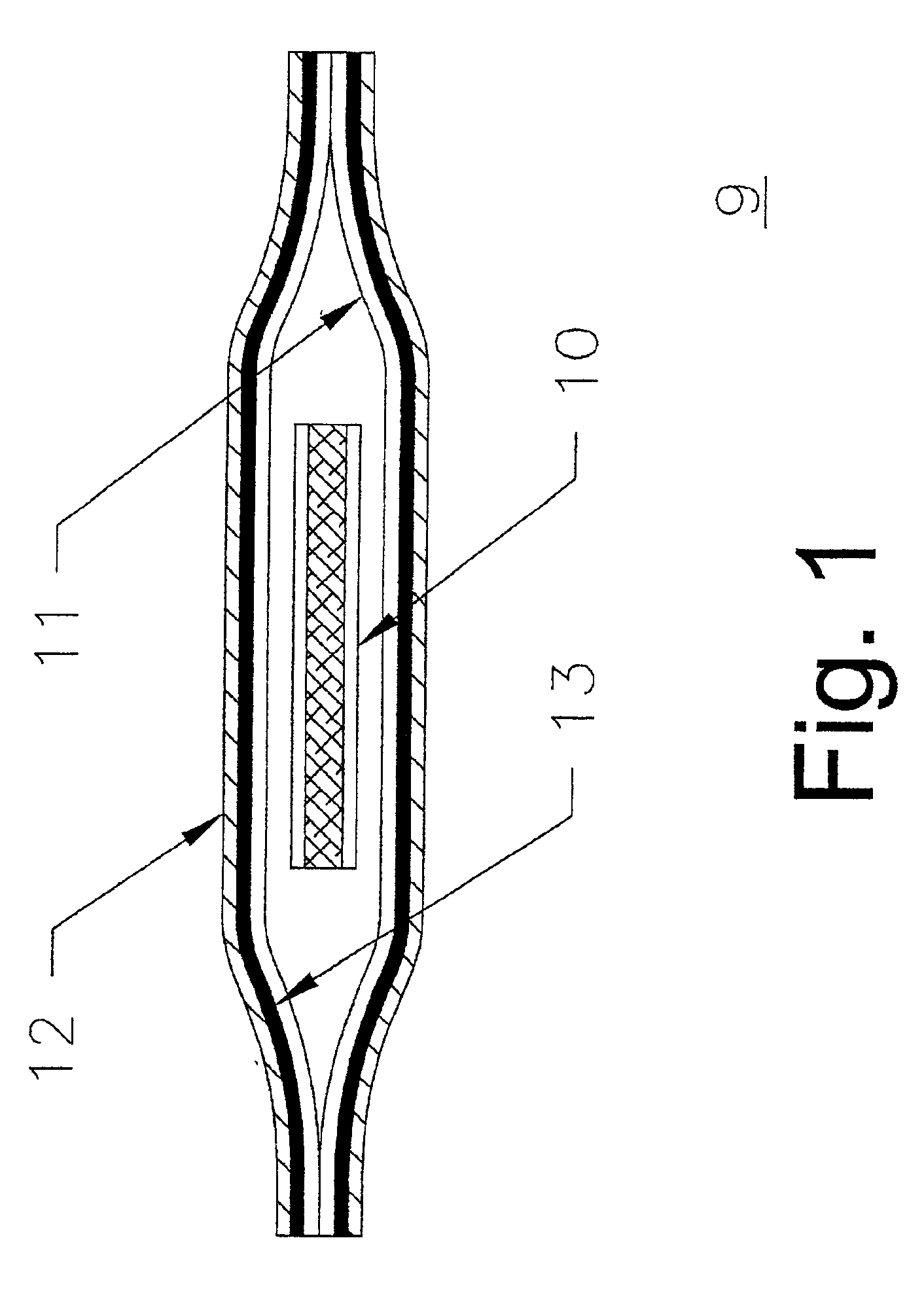
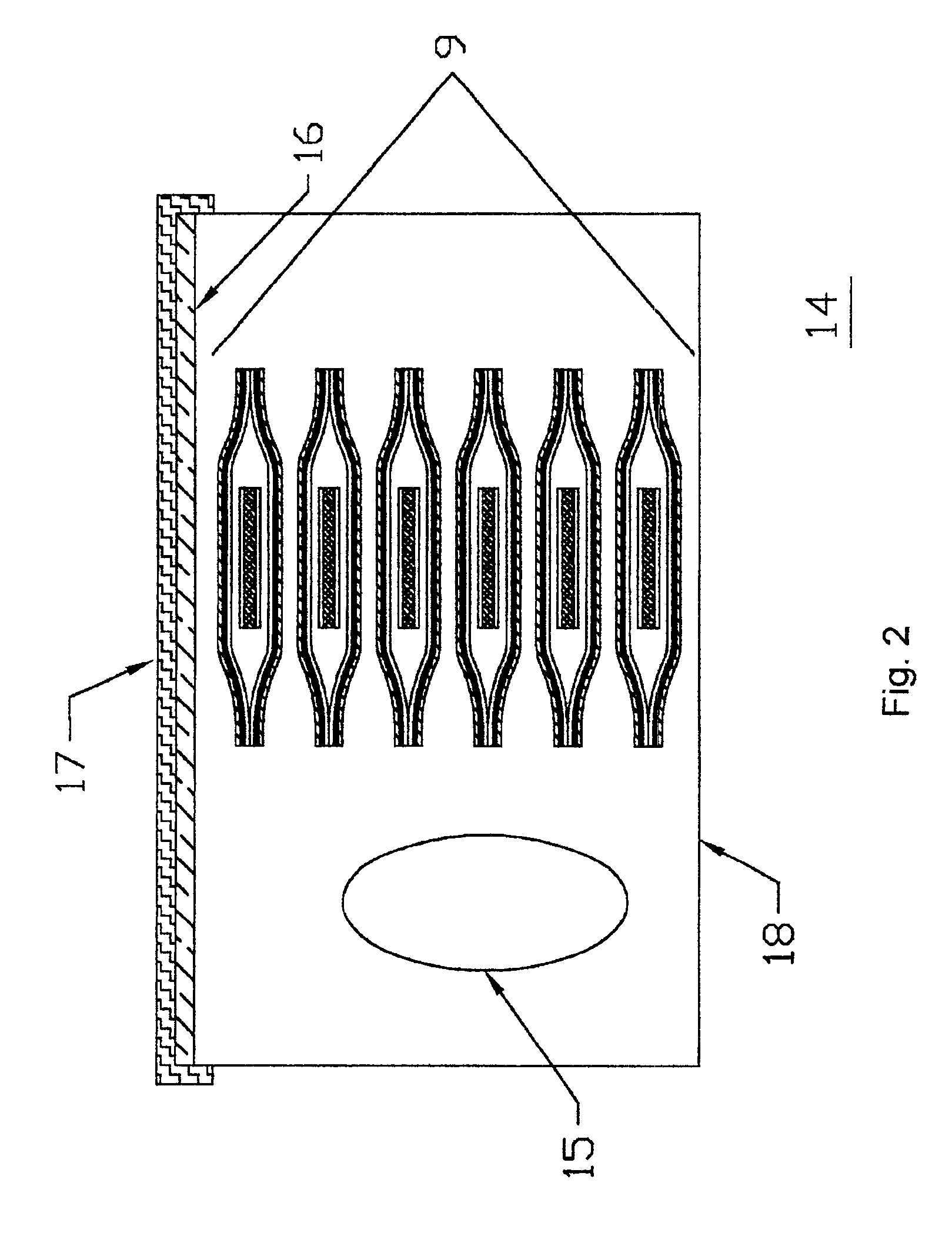
Packaging system for transdermal drug delivery systems
a technology of transdermal drug delivery and packaging system, which is applied in the direction of packaging foodstuffs, packaging goods, containers preventing decay, etc., can solve the problems of reducing the solubility of drugs in carrier composition, physical failure of packaging materials, and certain isomers that are actually deleterious rather than simply inactive or iner
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0070]A 1.25 mil film of Barex® 210 heat laminated to 0.35 mil aluminum foil. The aluminum foil was then bonded to 35# Kraft paper using an adhesive (laminate material manufactured by Richmond Technology, Redlands, Calif.).
example 2
[0071]A 1.25 mil film of Barex® 210 laminated with a polyester film using a urethane adhesive commercially available as 94035 and sold by Lawson Mardon (Shelbyville, Ky.).
example 3
[0072]A 1.25 mil film of Barex® 210 laminated with aluminum foil using an adhesive, which is then laminated to a polyester film using an adhesive, which is commercially available as 90580 and sold by Lawson Mardon.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com