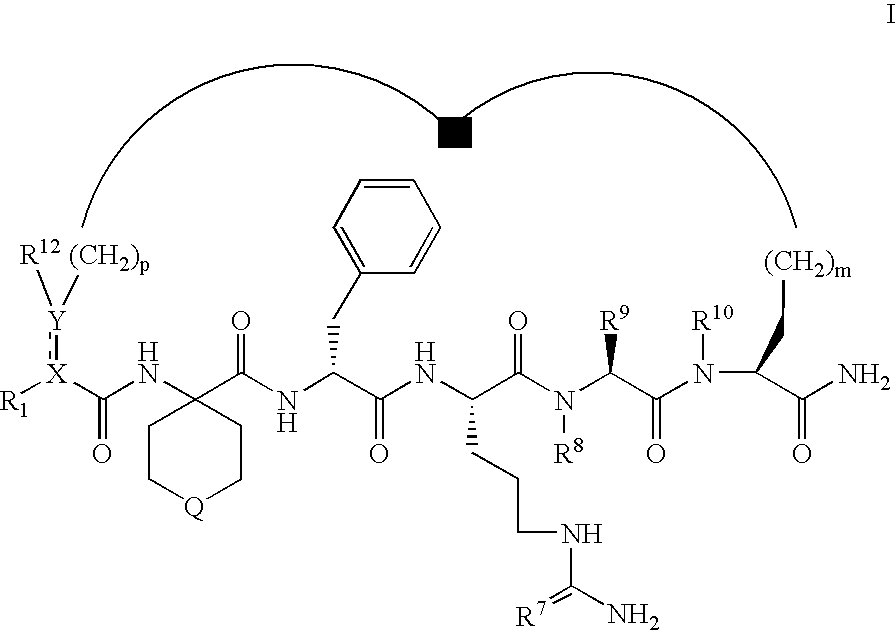
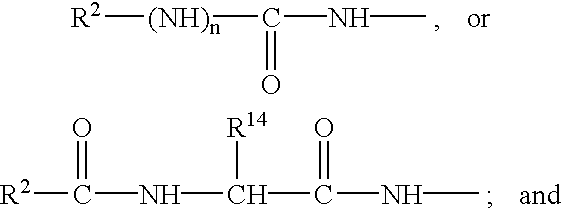
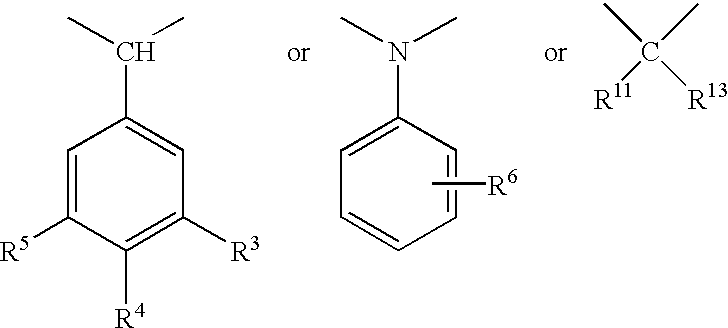
Selective cyclic peptides with melanocortin-4 receptor (MC4-R) agonist activity
a cyclic peptide and melanocortin-4 receptor technology, applied in the direction of peptide/protein ingredients, drug compositions, metabolic disorders, etc., can solve the problems of increasing the risk of skin cancer, adrenal cancer, and significant burden on the health care system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Fmoc-1-amino-4-phenylcyclohexane-1-carboxylic acid (Fmoc-Apc)
[0122]Step 1:
[0123]To a solution of 4-phenylcyclohexanone (10.0 g, 57.5 mmol) in ethanol (100 mL) and water (33 mL) in a glass pressure bottle, were added ammonium carbonate (33 g, 344 mmol, 6 equiv.) and potassium cyanide (5.6 g, 86.2 mmol, 1.5 equiv.). The mixture was heated at 80-90° C. for 24 hrs. The cooled reaction mixture was added to icy water (400 ml) and stirred vigorously for 30 min. The resulting precipitate was suction filtered, washed thoroughly with water and dried to yield the hydantoin A as a white solid (14.0 g, 100% yield). 1H NMR (DMSO-d6): 8.63 (s, 1H), 7.23-7.36 (m, 4), 7.15 (m, 1), 2.50 (m, 1H), 2.10 (m, 1H), 1.85 (d, 1H) and 1.55-1.80 (m, 6H).
Step 2:
[0124]The hydantoin A (10.0 g) was suspended in aqueous NaOH (6N, 350 mL) and heated at 130° C. for 2-3 days. Upon the completion of the hydrolysis, the reaction mixture was neutralized with conc. HCl to slightly acidic (pH˜6). The resul...
example 2
Preparation of Fmoc-1-amino4-(4-methoxyphenyl)cyclohexane-1-carboxylic acid (Fmoc-4-MeOApc-OH)
[0126]Step 1:
[0127]A solution of 4-(4-hydroxyphenyl)cyclohexanone (5.0 g, 26.3 mmol) in acetone (100 mL) was treated with K2CO3 (14.5 g, 105 mmol, 4 equiv) and iodomethane (4.9 mL, 11.2 g, 78.6 mmol, 3 equiv.). The reaction was heated at 65° C. overnight. After the solvent was removed, the residue was treated with H2O and extracted with EtOAc. The organic extracts were combined and washed with brine, dried over Na2SO4 and concentrated in vacuum to give the spectroscopically pure 4-(4-methoxyphenyl)-cyclohexanone (5.34 g, 100%). 1H NMR(CDCl3) 7.16 (dt, 2H), 6.87 (dt, 2H), 3.78 (s, 3H), 2.99 (tt, 1H), 2.47-2.53 (m, 4H), 2.20 (m, 2H) and 1.83-1.98 (m, 2H); MS (electrospray) m / e, 205 (M+1)+, Calcd for C13H16O2, 204.
Step 2:
[0128]To a solution of the 4-(4-methoxyphenyl)-cyclohexanone (3.86 g, 18.9 mmol) in ethanol (50 mL) and water (15 mL) in a glass pressure bottle, were added ammonium carbona...
example 3
Preparation of Fmoc-1-amino-4-(4-ethoxyphenyl)cyclohexane-1-carboxylic acid (Fmoc-4-EtOApc-OH)
[0131]Step 1:
[0132]A solution of 4-(4-hydroxyphenyl)cyclohexanone (5.0 g, 26.3 mmol) in acetone (100 mL) was treated with K2CO3 (14.5 g, 105 mmol, 4 equiv) and iodoethane (10.5 mL, 20.5 g, 131 mmol, 5 equiv.). The reaction was heated at 65° C. overnight. After the solvent was removed, the residue was treated with H2O and extracted with EtOAc. The organic extracts were combined and washed with brine, dried over Na2SO4 and concentrated in vacuum to give the spectroscopically pure 4-(4-ethoxyphenyl)-cyclohexanone (5.74 g, 100%). 1H NMR (CDCl3) 7.15 (dt, 2H), 6.86 (dt, 2H), 4.02 (q, 2H), 2.99 (tt, 1H), 2.46-2.54 (m, 4H), 2.16-2.24 (m, 2H), 1.83-2.00 (m, 2H) and 1.41 (t, 3H); MS (electrospray) m / e, 219 (M+1)+, Calcd for C14H18O2, 218.
Step 2:
[0133]To a solution of the 4-(4-ethoxyphenyl)-cyclohexanone (4.15 g, 19.01 mmol) in ethanol (50 mL) and water (15 mL) in a glass pressure bottle, were adde...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com