Culture chamber for biologicals
a technology of biologicals and chambers, applied in the field of culture chambers, can solve the problems of exacerbated cost of biologicals production in aseptic bioreactors, time and cost of purifying desired proteins from cell media, time and cost of cell culture protein manufacturing,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
second embodiment
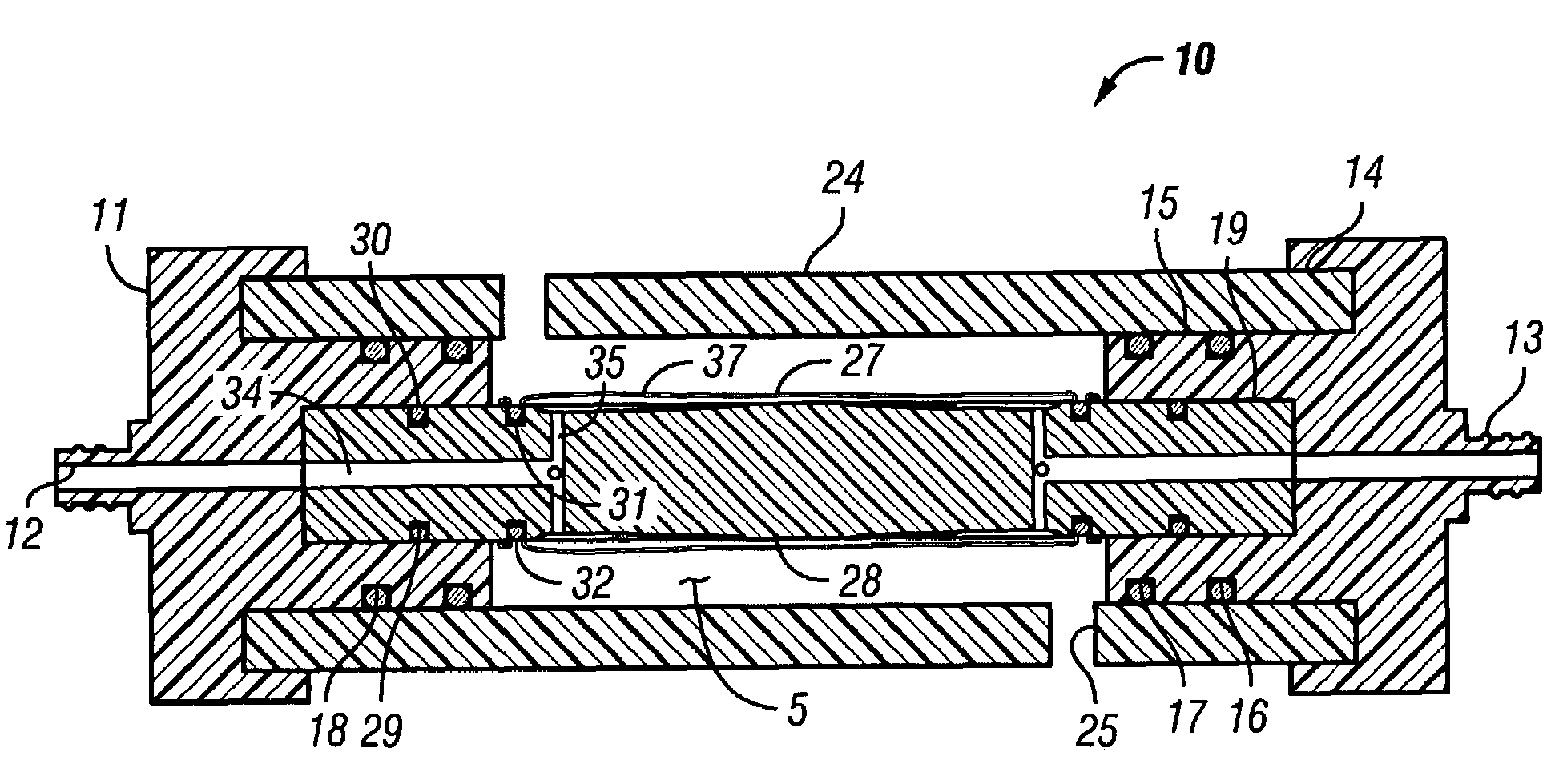
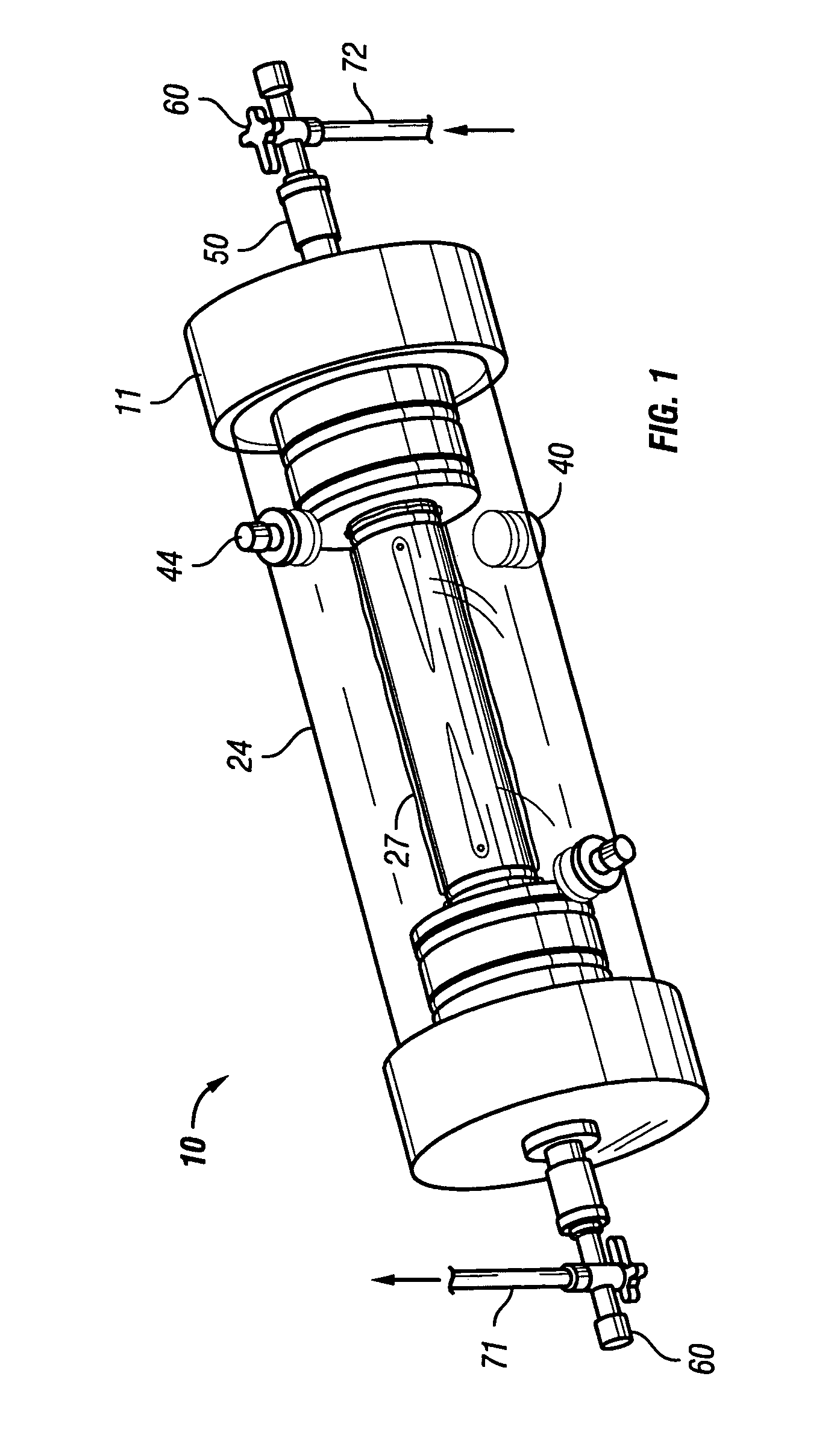
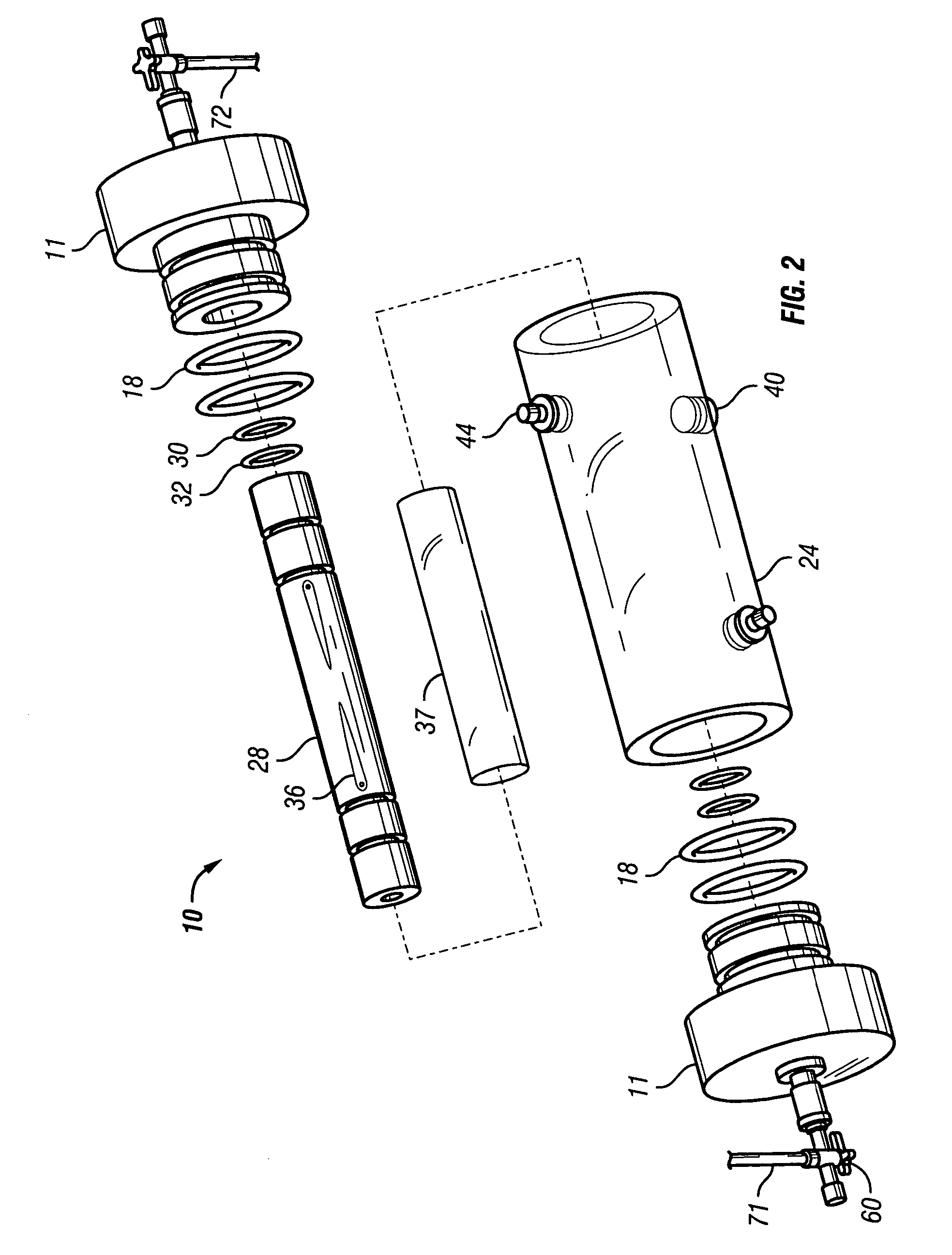
[0048]FIGS. 6-8 show a reusable culture chamber. The culture chamber 100 is similar in many ways to the first described culture chamber 10. Both culture chambers 10 and 100 use common components. In general, the culture chamber 100 is used for larger volume preparations than the culture chamber 10, given that the growth media was the same. If, on the other hand, the nutrients and / or waste products were slow to diffuse through the growth compartment 5 of culture chamber 10 due to use of a more restrictive molecular weight cut-off membrane, the multi-membrane carrier arrangement of culture chamber 100 might be used in a relatively small culture chamber.
[0049]As can be seen in FIGS. 6-8, two identical end pieces 111 seal to the ends of right circular cylindrical tubular sleeve 124 so that a growth compartment 105 is formed within the enclosed space. When in use for culturing cells and other biologicals, the culture chamber 100 is designed to be supported on and rotated by a roller driv...
third embodiment
[0054]A third embodiment, culture chamber 200, of a reusable culture chamber of the present invention is shown in FIGS. 9-11. Culture chamber 200 also uses multiple molecular weight cut-off membranes 37 mounted within the interior of the growth compartment 205. However, the structural mounting of the membranes for this embodiment is different than that used for the previously described embodiments. Although the culture chamber 200 and culture chamber 100 include different components, these two culture chambers are structurally similar and use several common components, including the sleeve 124, the end piece 111, the swivels 50, the stop cocks 60, and the tubings 72 and 71.
[0055]The culture chamber 200 is typically round in construction as it is designed to be supported on and rotated by a roller drive that rotates the chamber about its axis. Fluid-conducting swivels 50, stopcock valves 60, and fluid inlet tubing 72 and outlet tubing 71 are provided on the ends of the housing 200 so...
first embodiment
[0067]FIG. 13 shows a culture chamber 300 of the present invention having a disposable bioreactor bag assembly 301. As shown in FIG. 13, bag assembly 301 includes bag 320, two end pieces 305 for establishing fluid interconnections and positioning the bag assembly 301, and a centrally positioned membrane carrier assembly 27. Bag assembly 301 also includes one or more gas vent removal ports 425 and one or more fill ports 423.
[0068]All of the components of bag assembly 301 which contact the biological media and which are supplied to and removed from the bag assembly are biologically non-reactive, non-toxic and exhibit low protein binding properties. Bag assembly 301 and its constituent components are fused together with heat and pressure. However, the term fusing will be used with reference to joining elements of the bag assemblies of this invention where the elements are joined using heat, adhesive, ultrasonic welding, or other suitable means to effect the connections.
[0069]Preferably...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com