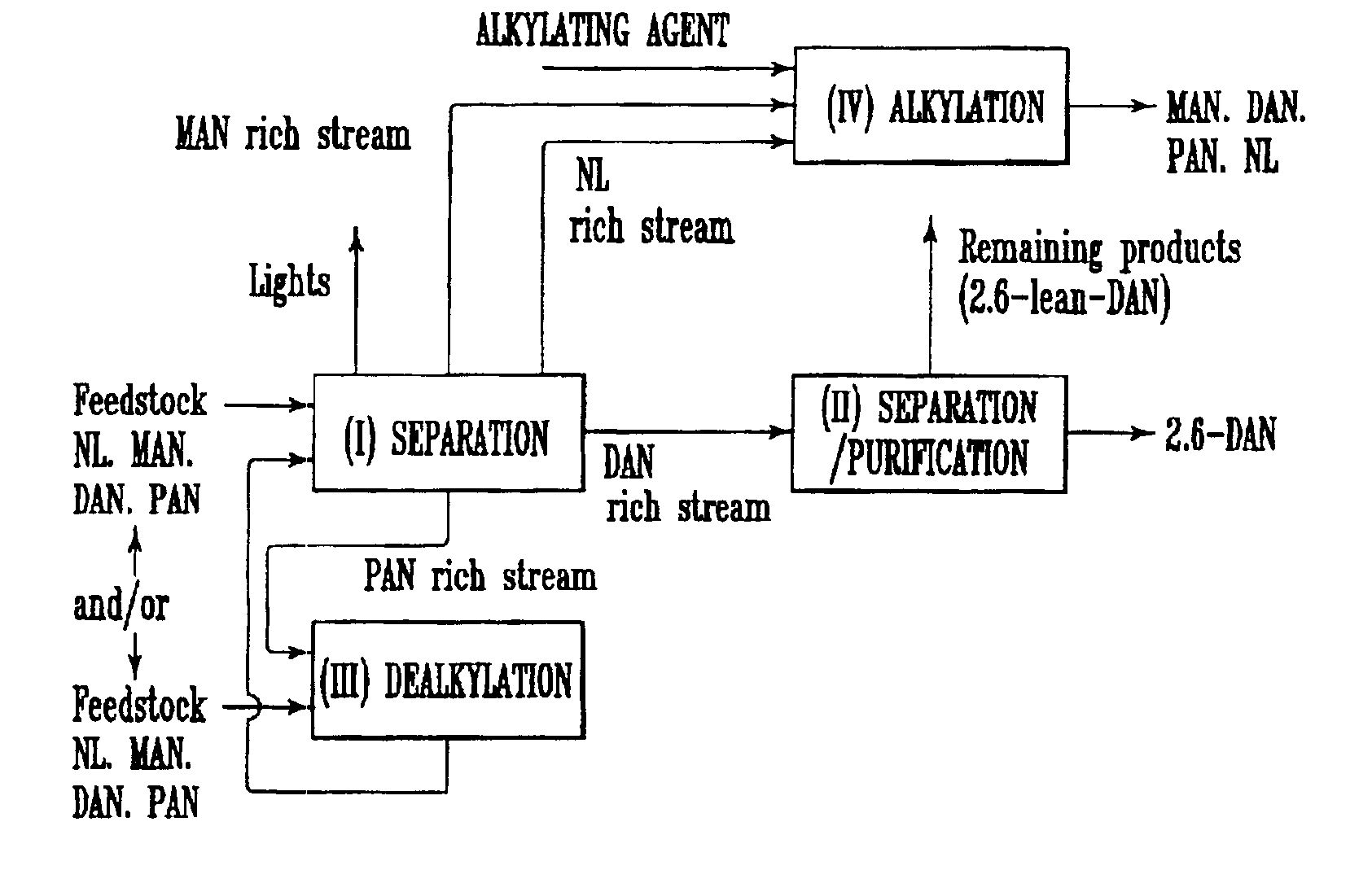
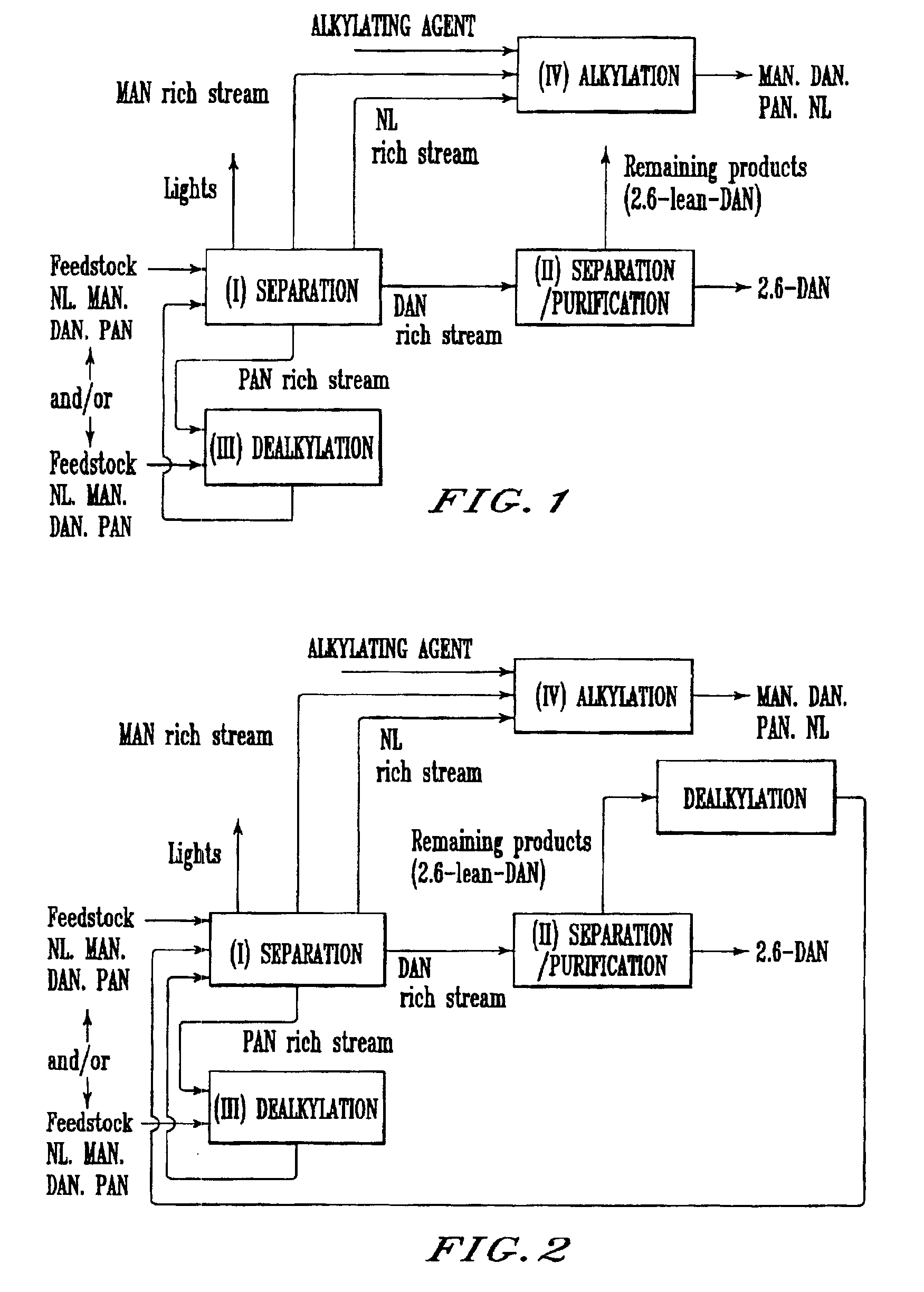
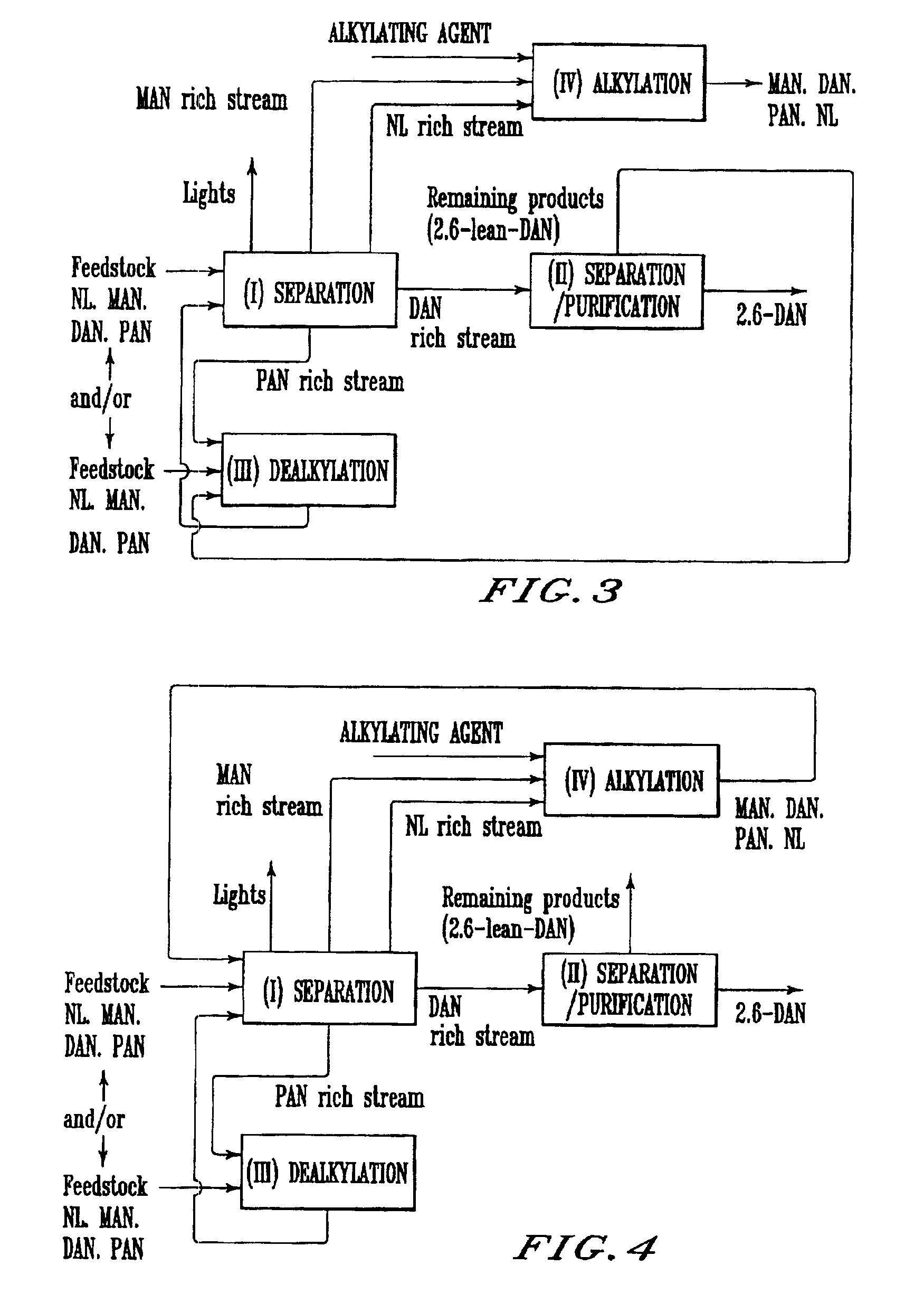
Process for preparing 2,6-dialkylnaphthalene
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Alkylation of MMN and Naphthalene
[0102]A 153 g amount of MCM-22 catalyst is charged into a tubular reactor (volume:370 cc). As a feedstock for alkylation, 1-MMN, 2-MMN and naphthalene are used, and mixed at a molar ratio of 2.2 of 2-MMN / 1-MMN, and a weight ratio of 3.0 of MMN's (1-MMN+2-MMN) / naphthalene.
[0103]Thereupon, the feedstock is supplied to the reactor (254 C, 5 kg / cm2) at a rate of 153.4 g / hr and 1.0 hr−1 in WHSV with a feed of hydrogen at the rate of 1.8 ft3 / hr. Four hours later, methanol, as an alkylating agent, is introduced into the reactor at 35.5 g / hr, and alkylation is conducted for 20 hours. The product obtained is analyzed by gas chromatography, and the results are summarized in Table 1.
[0104]
TABLE 1(Alkylation of Monomethylnaphthalene and Naphthalene)Before ReactionAfter ReactionComponent (wt %)dimethylnaphthalene017.192,6-DMN01.722,7-DMN01.20other isomers014.27monomethylnaphthalene73.6360.102-MMN50.5540.321-MMN23.0819.78naphthalene25.2818.67other component1.003.9...
example 2
[0106]153 g of MCM-22 were charged in the tubular reactor (volume: 370 cc). As a feedstock for alkylation, 1-MMN (purity 95.5%) and 2-MMN (purity 96.6%) were used, and mixed at the molar ratio of 2.2 of 2-MMN / 1-MMN. Feedstock was supplied in the reactor (350° C.) at the rate of 76.7 g / hr and 0.5 hr−1 in WHSV for 4 hours. Thereafter, methanol was started to be supplied in the reactor at the rate of 17.3 g / hr and the reaction was proceeded for 20 hours. The obtained product was analyzed by gas chromatography, and the result is summarized in Table 2.
[0107]
TABLE 2(Alkylation)before reactionafter reactioncomponent (wt %)dimethylnaphthalene035.452,6-DMN05.122,7-DMN04.44other isomers025.89monomethylnaphthalene98.6641.162-MMN67.6128.841-MMN31.0512.32naphthalene00.19other component (mainly PAN)1.5323.20evaluation2-MMN / 1-MMN2.22.3MMN conversion (%)—58.282,6-DMN / total DMN (%)—14.452,6-DMN / 2,7-DMN—1.16
[0108]As can be seen from Table 2, the ratio of 2,6-DMN / 2,7-DMN is over 1.1 and th...
example 3
(Alkylation and Distillation)
[0109]Alkylation of MMN and naphthalene has been carried out for several months in the same manner described in Example 1 and about 400 kg of the product is collected. Distillation of the product is carried out by using a batch type distillation tower with a packed column. A number of theoretical trays of the tower is expected to be at least 50. The operation pressure at the top of the column is controlled between 15 and 36 Torr and distillation proceeds at a reflux ratio of 50 to 75.
[0110]The product is separated into 17 fractions by differences in boiling points as shown in Table 3.
[0111]
TABLE 3(Alkylation and Distillation)AmountDMN2,6-DMN(kg)concentration (%)concentration (%)Fraction-1-10270.80.0not analyzedFraction-1130.90.5not analyzedFraction-128.838.9not analyzedFraction-1311.064.811.2 Fraction-146.392.325.4 Fraction-1515.799.64.3Fraction-164.898.70.0Fraction-175.341.60.0Residue21.20.00.0
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap