Methods for stable transduction of cells with viral vectors
A technology of lentiviral vectors and cells, which is applied in the direction of viruses/bacteriophages, viruses, and the use of vectors to introduce foreign genetic materials, etc., which can solve the problems that cells are not suitable for human clinical transplantation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] Preparation of naive CD4+ T cells
[0108] With a slight modification of the standard method, peripheral blood CD4+ T cells were isolated. Specifically, confounding monocytes were removed using the adherence method. Non-adherent cells were contacted with magnetic beads coated with anti-CD4+ antibody to select for CD4+ cells. Take out the magnetic beads and separate the CD4+ cells.
[0109] The purity of highly purified CD4+ cells is as high as 90% through flow cytometry test.
Embodiment 2
[0111] Naive CD4+ T cells were transduced with cell surface binding molecules at different contact times.
[0112] Transduction prior to cell surface binding
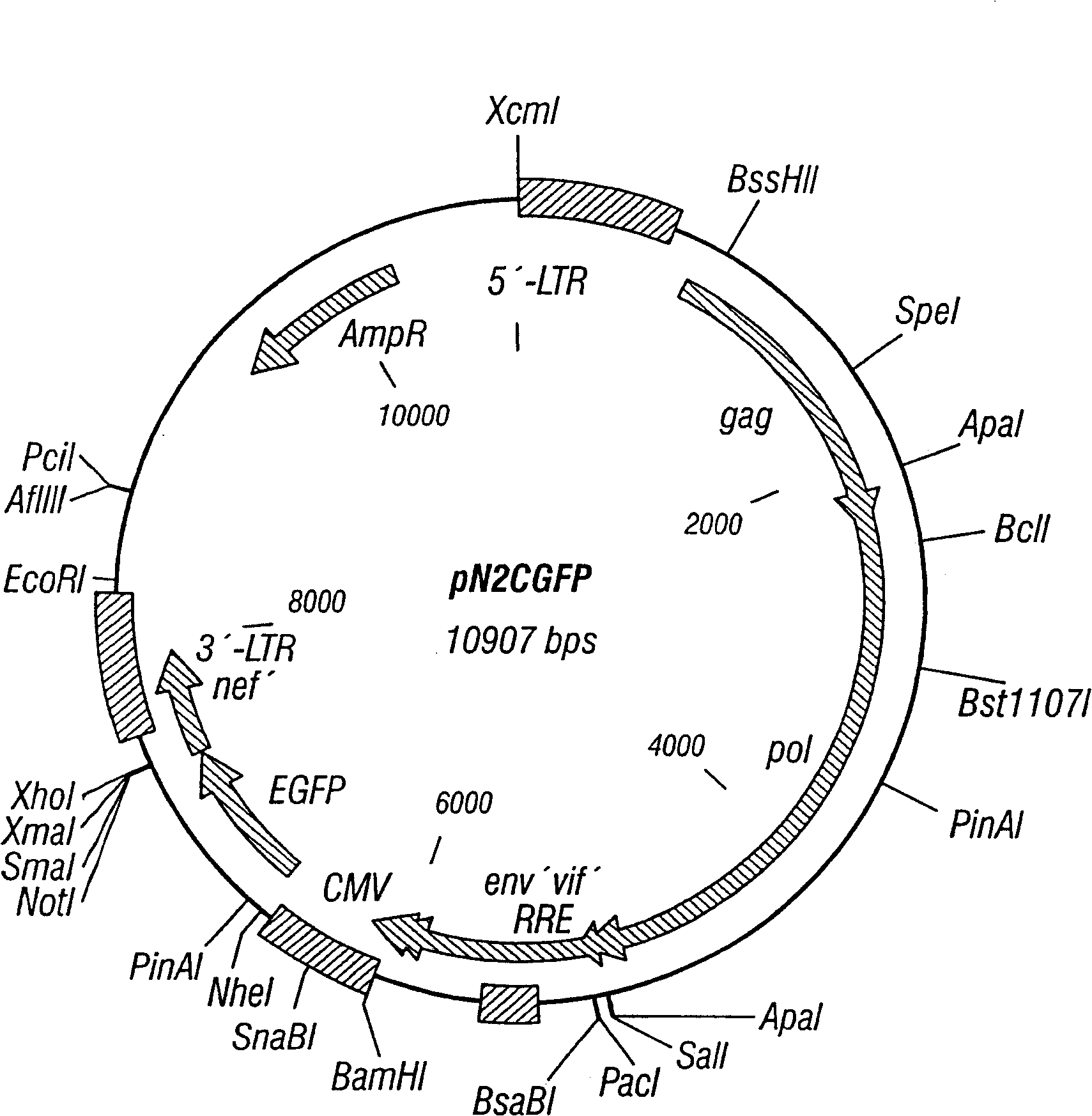
[0113] Naive CD4+ cells (about 500,000 cells) were co-cultured with pN2cGFP at MOI 20 for 24 hours, then added microbeads coated with αCD3 and αCD28, and cultured for another 7 days. Figure 1 shows the map of pN2cGFP. Transduction after cell surface binding
[0114] Naive CD4+ cells (approximately 500,000) were co-cultured with αCD3 and αCD28-coated microbeads for 24 hours, then pN2cGFP was added to the culture at an MOI of 20, and co-culture was continued for 24 hours. Then, they were cultured for 7 days in a medium containing microbeads but no carrier to wash away excess carrier.
[0115] Simultaneous cell surface binding and transduction
[0116] Naive CD4+ cells (~500,000) were co-cultured at MOI 20 with pN2cGFP for 24 hours in the presence of microbeads coated with αCD3 and αCD28. Then, they were cultured fo...
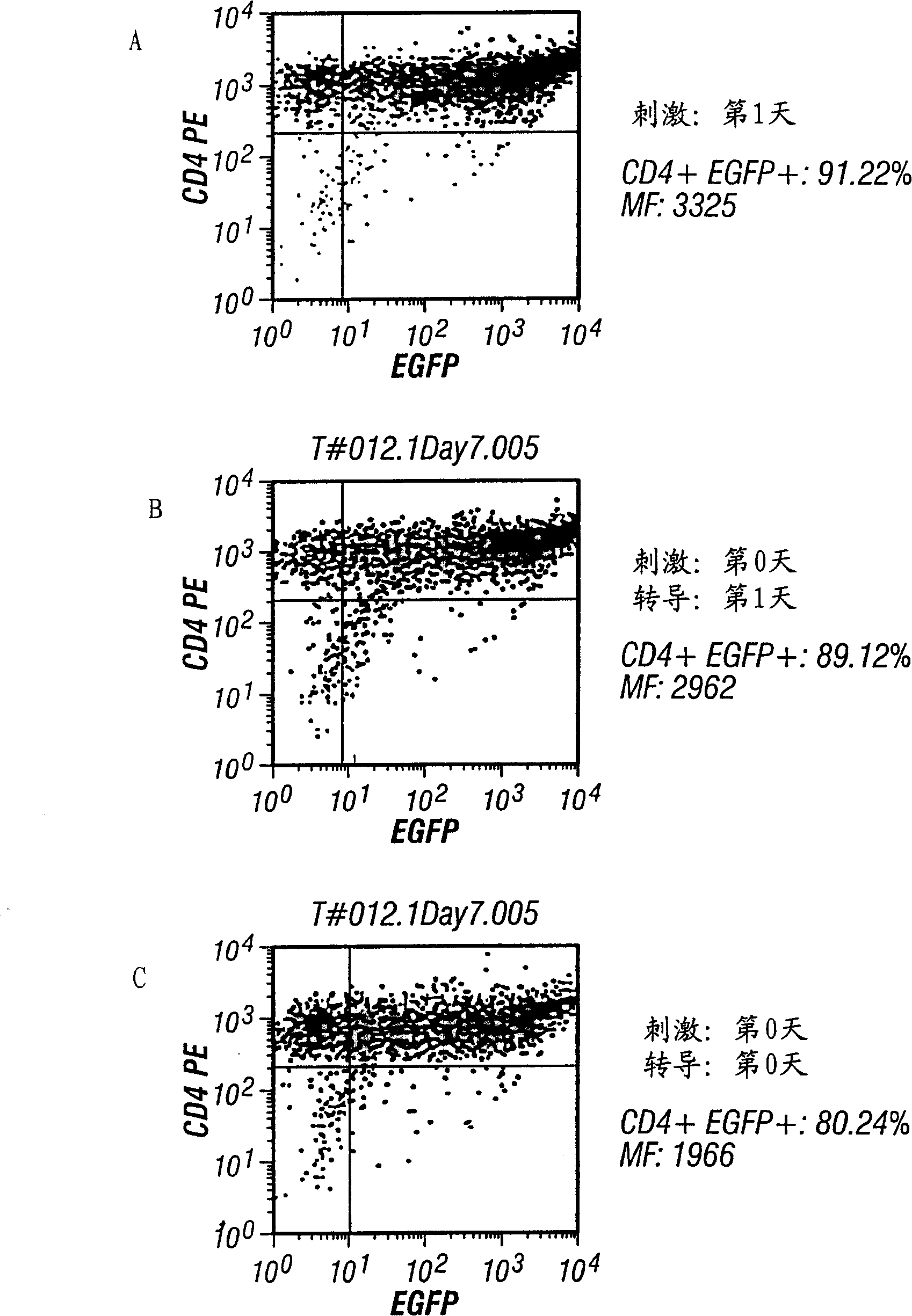
Embodiment III
[0121] Post-transduction analysis
[0122] After transduction, and after 7 or 14 days in culture, cells were analyzed for CD4+ and / or green fluorescent protein (GFP) by flow cytometry.
[0123] A comparison of the above three transduction schemes can be seen figure 2 . After being transduced with pN2cGFP at MOI 20, it was contacted with αCD3 and αCD28 immobilized on microbeads, and the transduction rate was about 91%. The efficiency of bead contact followed by transduction was about 89%, and the transduction rate performed simultaneously was about 80%. In this experiment, CD4+ T cells were selected by monocyte adhesion removal method, CD14MACS removal method and CD4MACS enrichment method. Antibody immobilization was as follows. Contact with support at 37°C and 5% CO 2 under conditions. The culture condition is 500,000 CD4+T cells / ml, Yssel's medium containing 2% human serum albumin. FACS analysis was performed on day 7 after selection. MF indicates mean fluorescence. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com