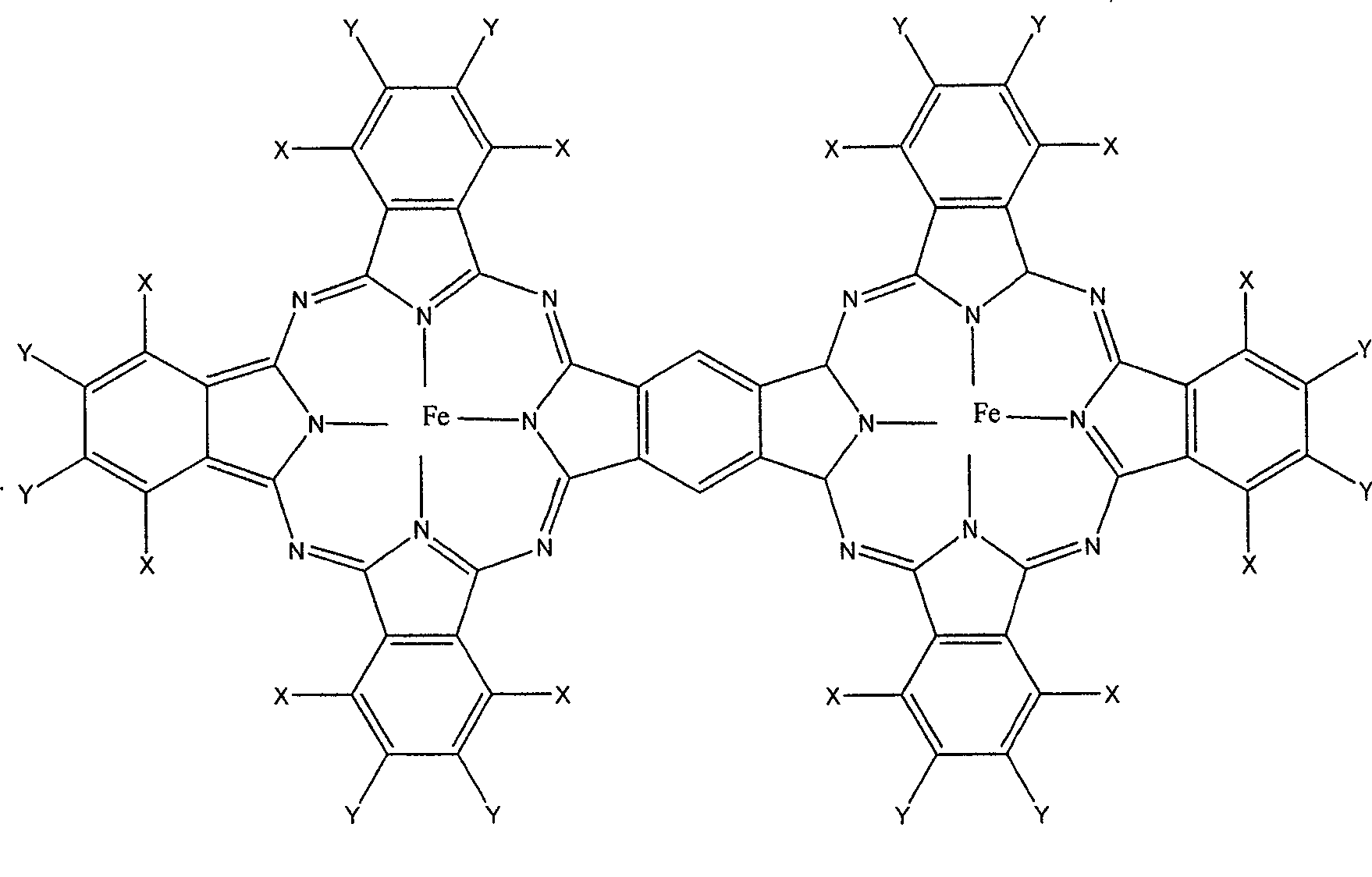
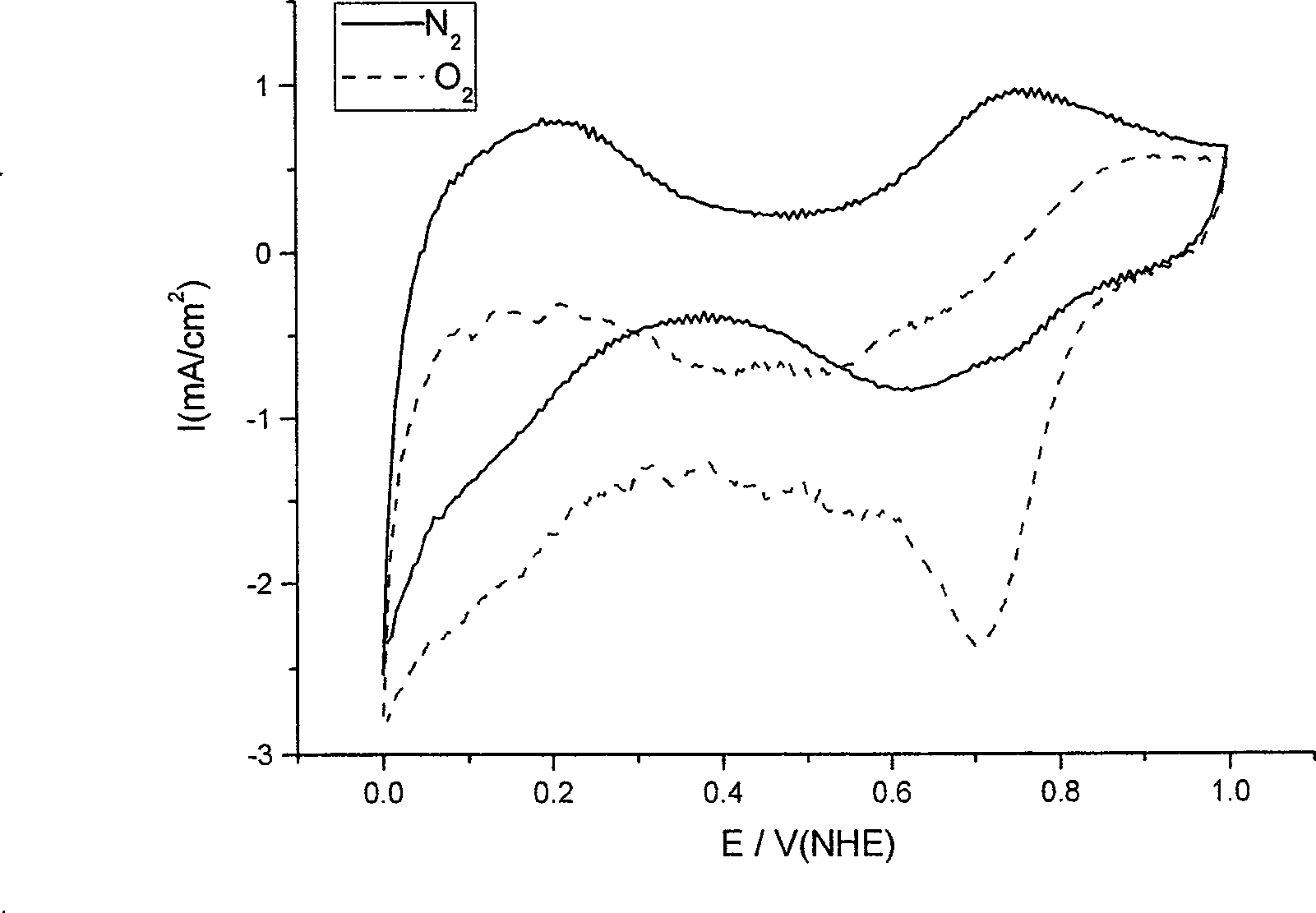
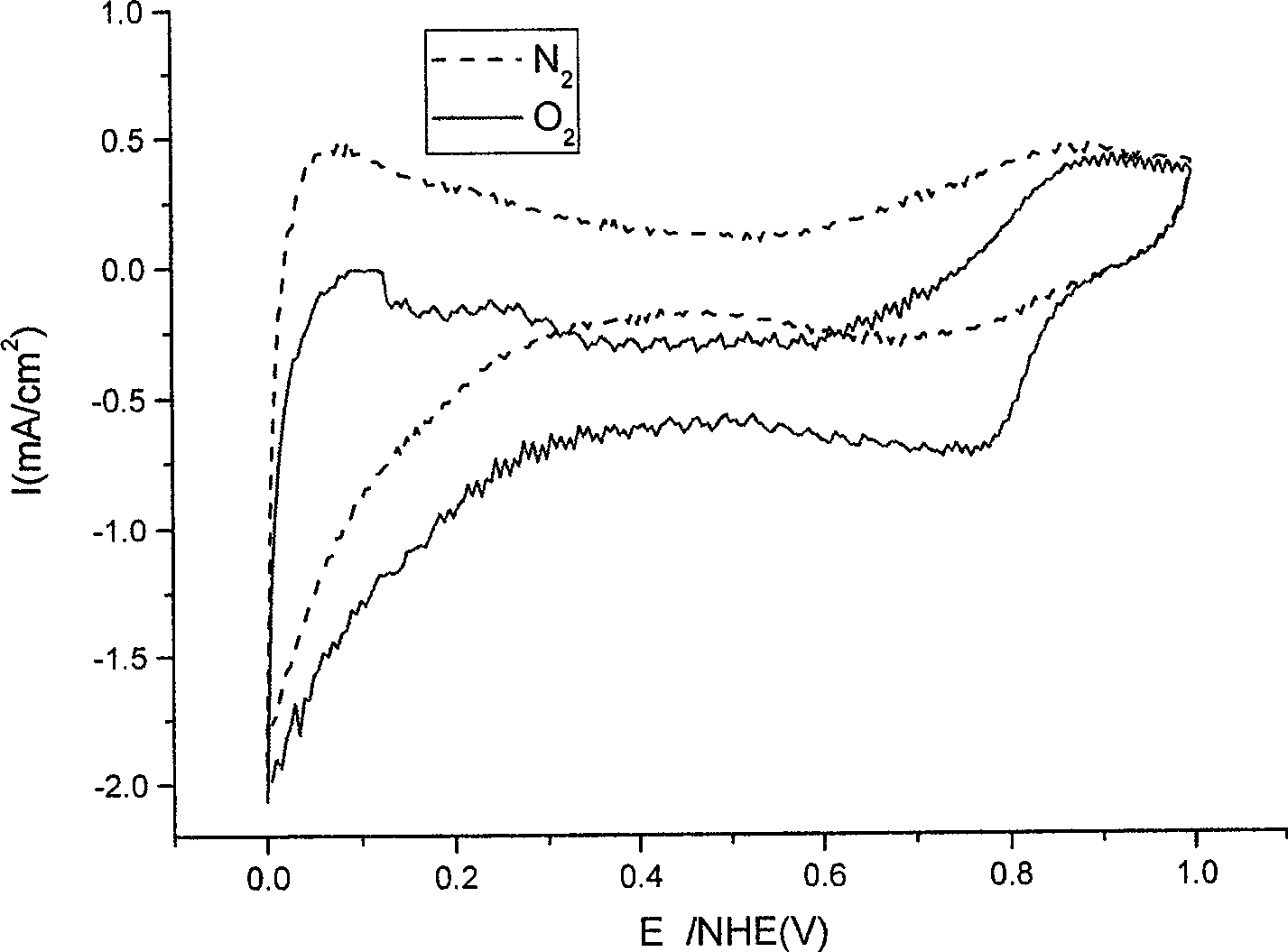
Halogen-substituted binuclear phthalocyanine ferrite reduction catacolyst and preparing method thereof
A nuclear iron phthalocyanine and catalyst technology, applied in the field of electrocatalysis, can solve problems such as poor stability, and achieve the effects of good stability, high catalytic activity, and efficient oxygen reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] (1) 6.51g3.6-dichlorophthalic anhydride, 1.10g pyromellitic anhydride, 1.98gFeCl 2 4H 2 O, 9.47g of urea and 0.25g of ammonium molybdate were mixed evenly and finely ground. After the mixture was heated at 190°C for 1 hour, the reaction was continued at 250°C for 2 hours. The product was successively washed with ammonia solution, dilute hydrochloric acid and water, and then dried. The crude product was extracted and washed with ethanol and tetrahydrofuran until it was colorless, and further purified by silica gel chromatography (N,N-dimethylformamide as the eluent) to obtain the dark blue target product dodecachloro-substituted dinuclear phthalocyanine iron (II ). UV-Vis (DMSO as a solution): λ max 662nm. 1 HNMR (DSMO-d 8 ): δ11.3~11.8 (2H, aroma). Elemental Analysis: Calculated for C, 50.07; H, 13.58; N, 15.10; Fe, 7.53. Found C, 50.01; H, 13.55; N, 15.12; Fe, 7.60. Mass Spectrum (LDI-TOF-MS) Found (Calcd): M / e=1483.6 (1483.1) [M + ]
Embodiment 2
[0021] 6.60g tetrafluorophthalic anhydride, 1.10g pyromellitic anhydride, 1.98g FeCl 2 4H 2 O, 9.47g of urea and 0.25g of ammonium molybdate were mixed evenly and finely ground. After the mixture was heated at 110°C for 1.5h, the reaction was continued at 220°C for 3h. The product was washed with ammonia solution, dilute hydrochloric acid and water in turn, and then dried. The crude product was extracted and washed with ethanol and tetrahydrofuran until it was colorless, and further purified by silica gel chromatography (N,N-dimethylformamide as the eluent) to obtain the dark blue target product Tetrafluoro-substituted dinuclear iron phthalocyanine ( II). UV-Vis (DMSO as a solution): λ max 651nm. 1 HNMR (DSMO-d 8 ): δ 10.5 to 10.8 (2H, aroma). Elemental analysis: calculated value C: 49.06, H: 13.31, N: 14.30, Fe: 7.37; found value C: 49.02, H: 13.40, N: 14.26, Fe: 7.42. Mass Spectrum (LDI-TOF-MS) Found (Calcd): M / e=1514.2 (1513.6) [M + ]
[0022] The target product obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com