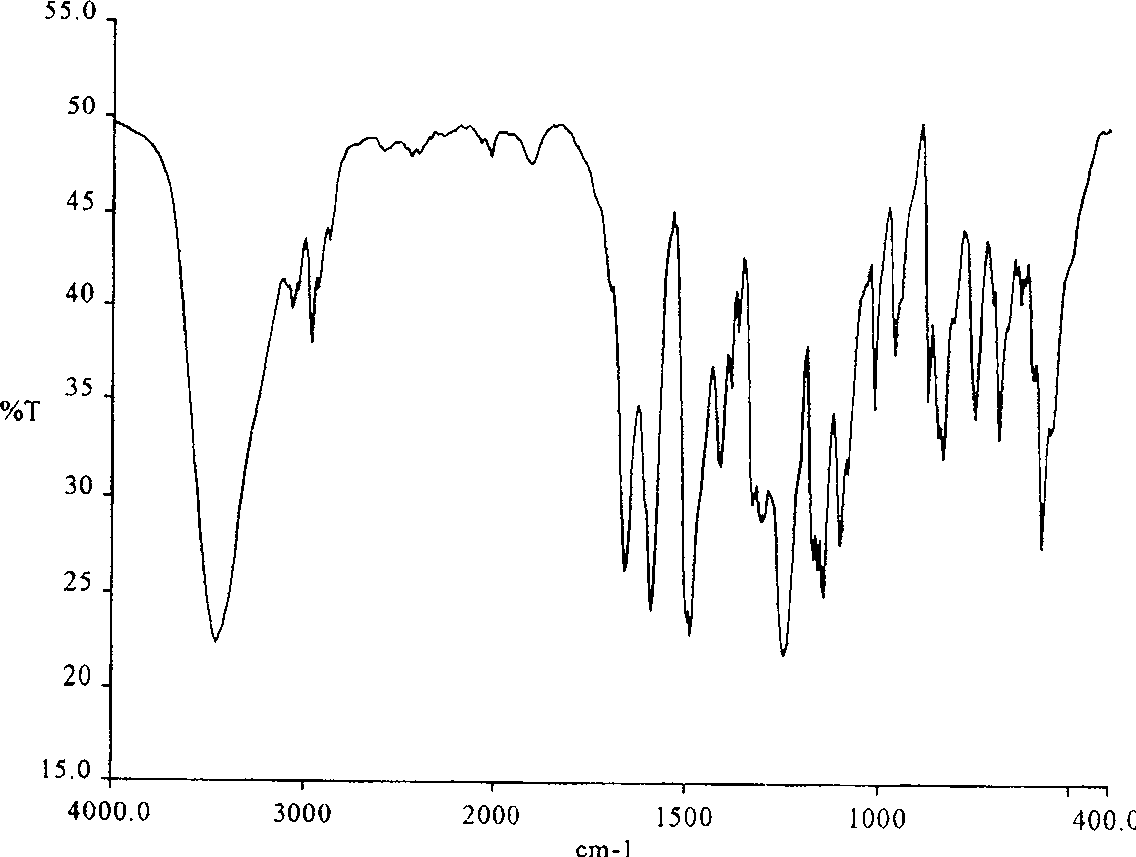
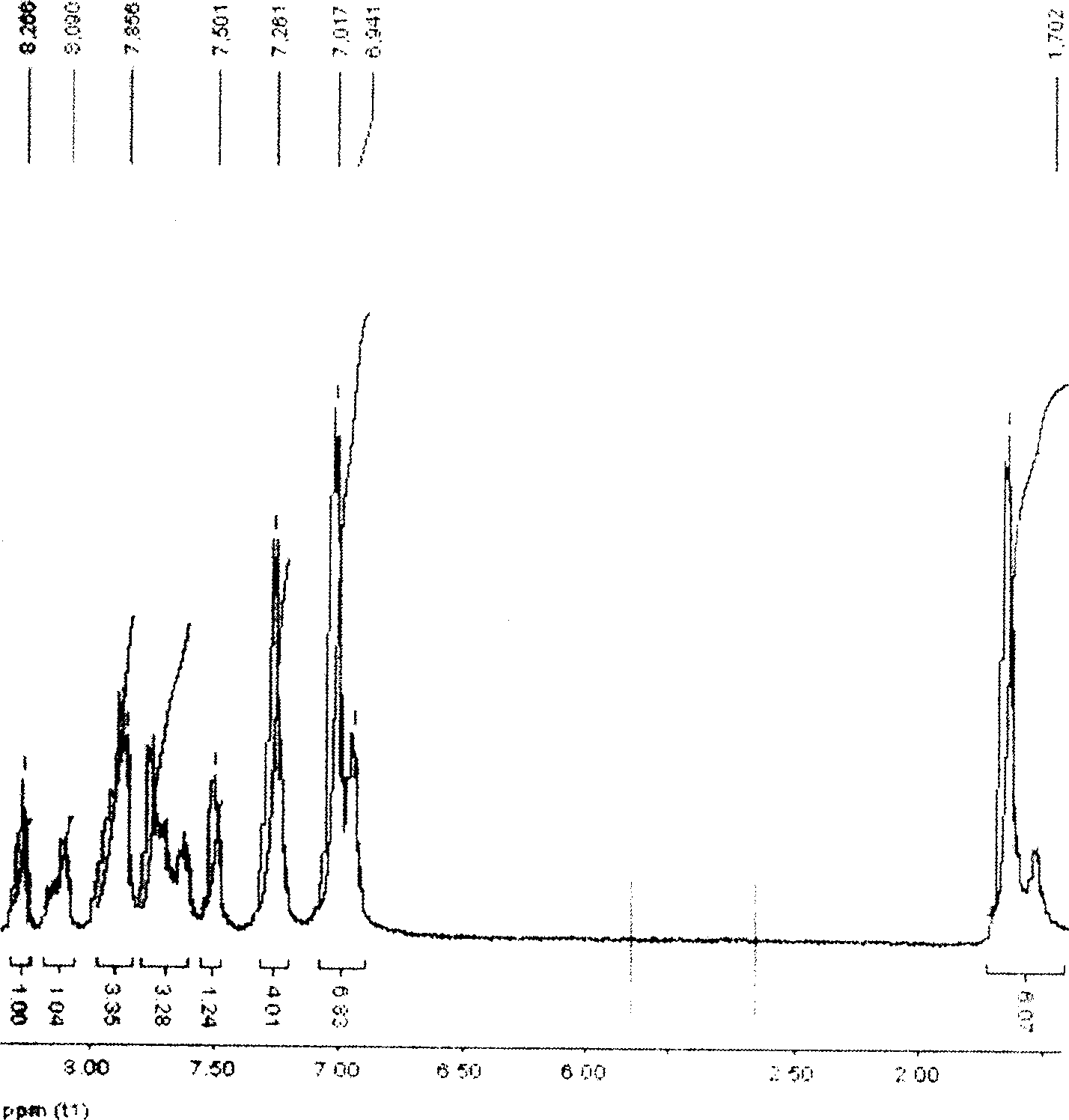
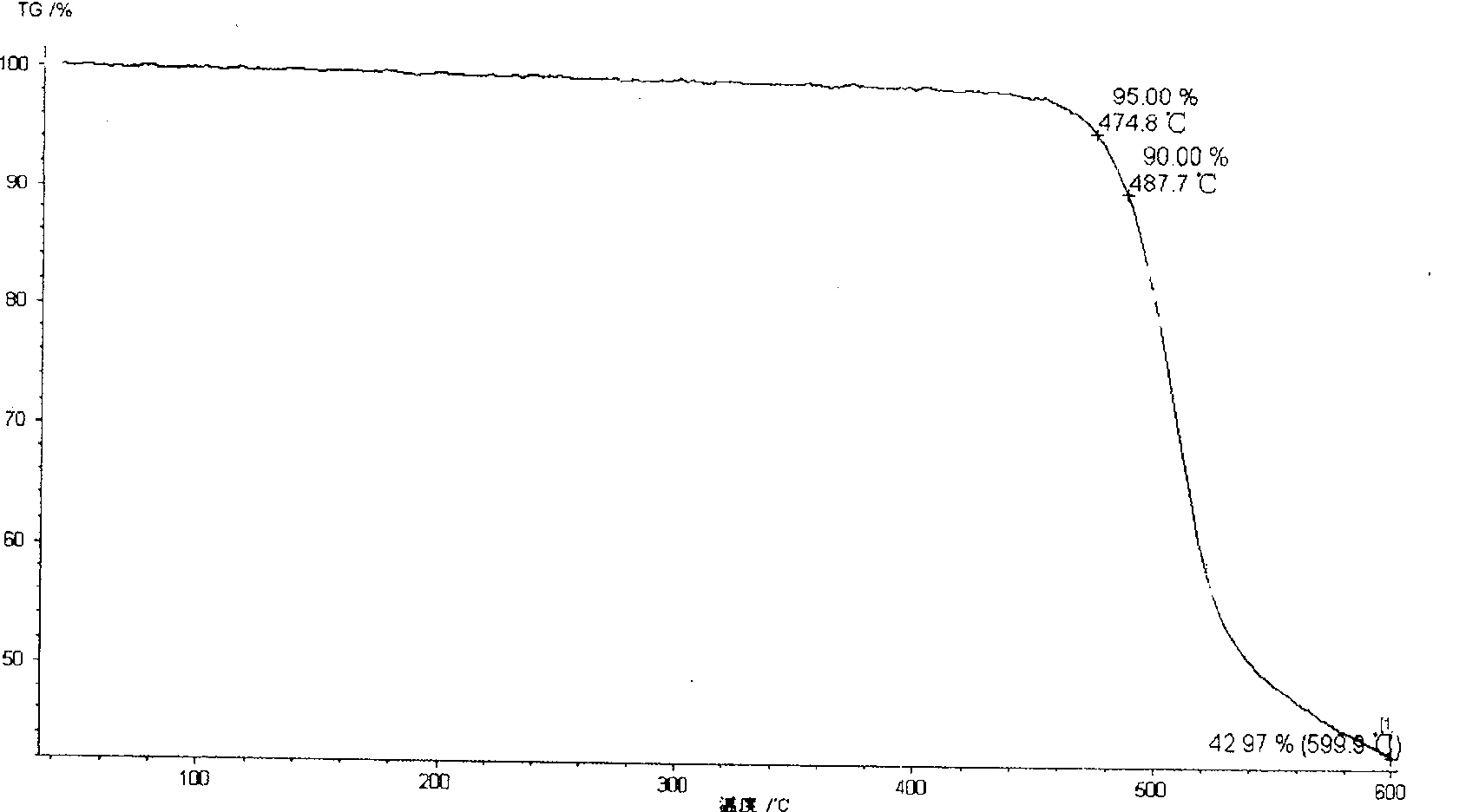
Method of synthesizing poly(phthalazinone ether sulfone ketone) containing interposition asymmetric sulfone ketone structure at backbone
A polyaryl ether sulfone ketone and a synthesis method technology, applied in the field of organic polymer compounds, can solve the problems of high cost and difficulty in industrialization, and achieve the effects of low price, improved solubility and processability, and superior performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0032] Synthesis of Example 1 PPEKS
[0033] In a four-necked flask with a stirrer, a thermometer, a reflux condensing device, a water separator, and a nitrogen pipe, under nitrogen protection, add (26.1 g) of 1-(p-chlorobenzoyl chloride)-3- (p-Chlorobenzenesulfonyl chloride) benzene and (15.2g) of bisphenol A, (16.4g) of anhydrous potassium carbonate, add 100ml of DMAC, 75ml of toluene, heat to reflux, start to separate water at about 130 ° C, separate After the water is complete, the temperature is gradually raised to 155°C, and the color of the reaction system changes from yellow to brown at this time. After reacting at this temperature for more than 10 minutes, cool down to about 130°C and then add toluene to ensure that the water is completely taken away, then slowly raise the temperature to 155°C for polycondensation reaction, and reach the predetermined viscosity when the reaction liquid becomes viscous (0.60 ~1.30dL / g), stop the reaction, lower the temperature to 100°...
example 2
[0034] Synthesis of Example 2 PPEKS
[0035] With a stirrer, a thermometer, a reflux condensing device, a water separator, and a four-necked flask with a nitrogen pipe, under nitrogen protection, 26.1g of 1-(p-chlorobenzoyl chloride)-3-(p-chlorobenzoyl chloride) was added successively. Chlorobenzenesulfonyl chloride) benzene and 15.2g of bisphenol A, 15.5g of anhydrous potassium carbonate, add 100ml of sulfolane and 75ml of toluene, heat to reflux, start water separation at about 130°C, and gradually heat up to 160°C after water separation is complete , the color of the reaction system changed from yellow to brown. After reacting at this temperature for more than 10 minutes, lower the temperature to about 130°C and then add toluene to ensure that the water is completely taken away, and then slowly raise the temperature to 155°C for polycondensation reaction. Others are the same as example 1.
example 3
[0036] Synthesis of Example 3 PPEKS
[0037] With a stirrer, a thermometer, a reflux condensing device, a water separator, and a four-necked flask with a nitrogen pipe, under nitrogen protection, 26.1g of 1-(p-chlorobenzoyl chloride)-3-(p-chlorobenzoyl chloride) was added successively. Chlorobenzenesulfonyl chloride) benzene and 15.2g of bisphenol A, 15.2g of anhydrous potassium carbonate, add 164.7ml of sulfolane, 75ml of xylene, heat to reflux, start water separation at about 150°C, and gradually heat up to At 165°C, the color of the reaction system changed from yellow to brown. After reacting at this temperature for more than 10 minutes, cool down to about 150°C and add xylene to ensure that the water is completely taken away, and then slowly raise the temperature to 155°C for polycondensation reaction. Others are the same as example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com