Sterilizing pretest pack for endoscope
A sterilizing test and endoscope technology, which is applied in toilet sanitary equipment, disinfection, construction, etc., can solve the problems of impossible to confirm the bactericidal effect of endoscope, difficult operation, large volume, etc., and achieve simple and effective bactericidal, Reliable sterilization effect, simple and confirmed effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
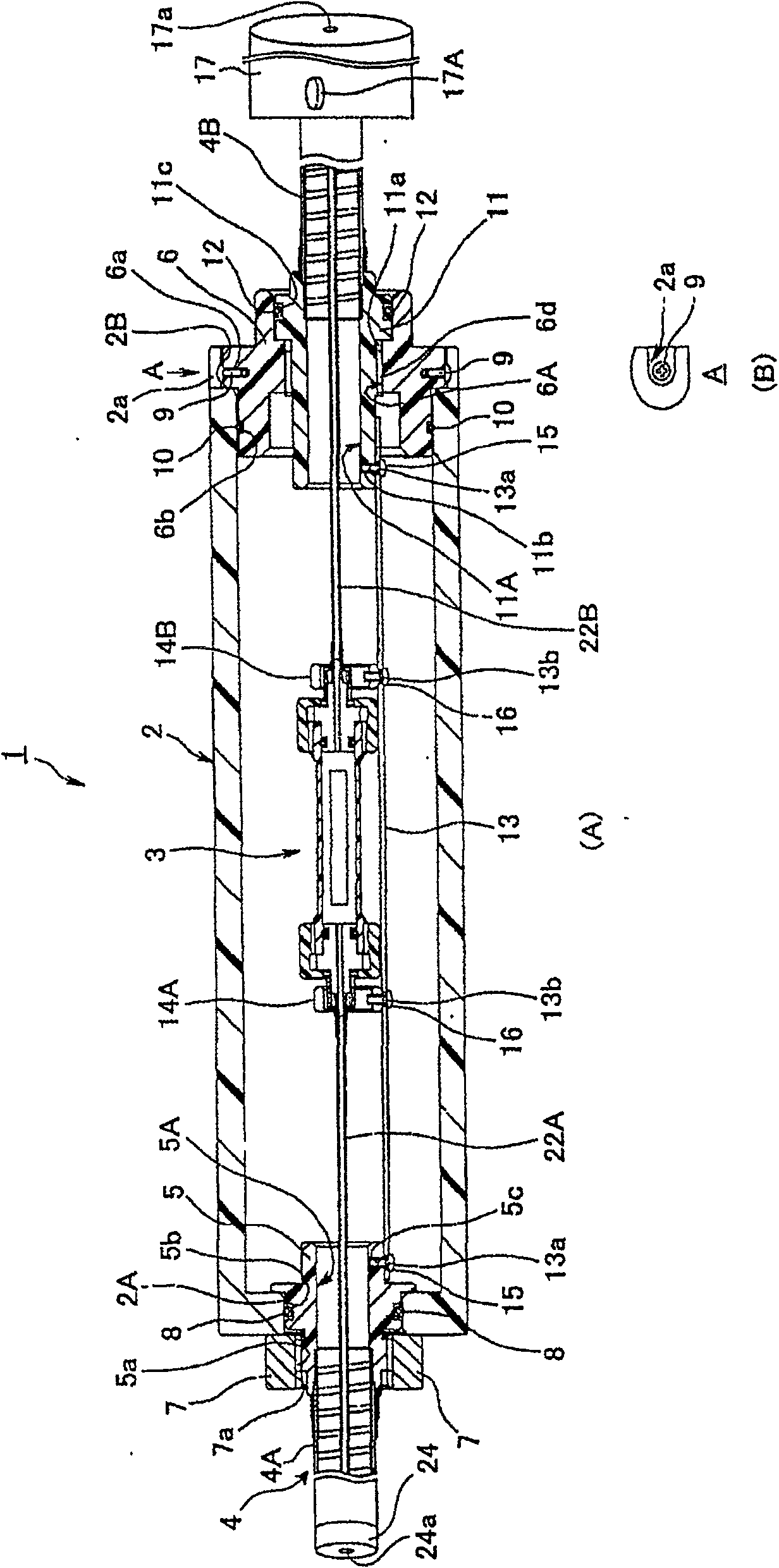
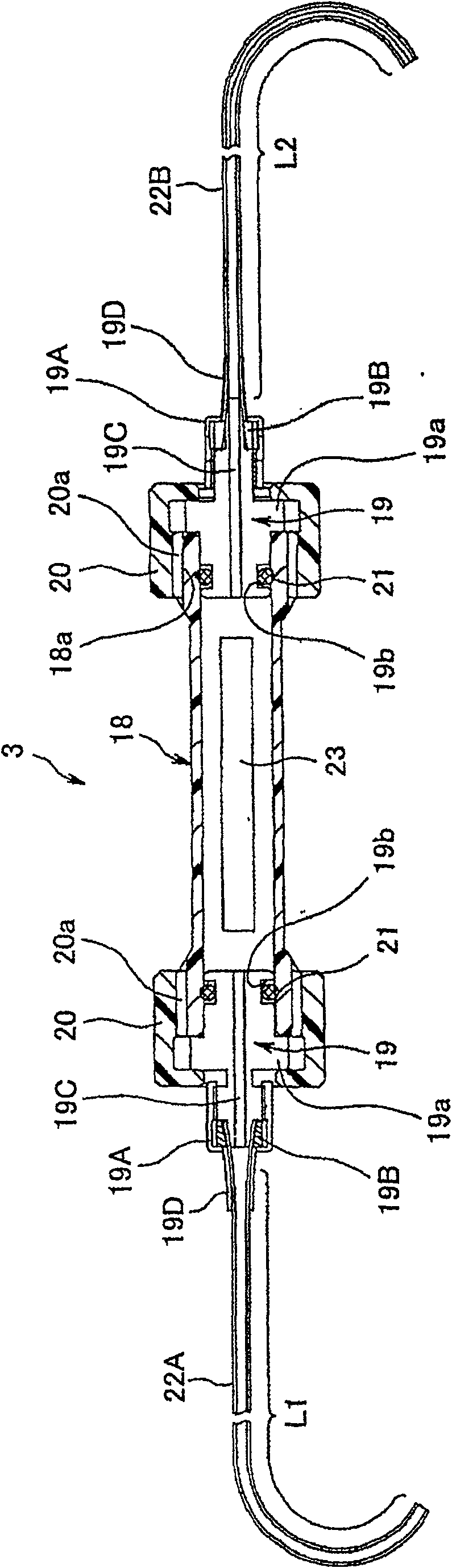
[0061] figure 1 and figure 2 Representing an embodiment of the endoscope sterilization test assembly of the present invention, figure 1 (A) shows a cross-sectional view of the overall structure of the endoscope sterilization test assembly, figure 1 (B) means from figure 1 (A) The figure of the connection part seen from the direction of the arrow A, figure 2 means housed in figure 1 Cross-sectional view of the inner shell structure within the outer shell.
[0062] Such as figure 1 As shown in (A), the sterilization test assembly 1 for an endoscope has the following parts: an outer shell 2; an inner shell 3 detachably accommodated in the outer shell 2; first and second hoses 4A, 4B disposed on the outer shell. Both sides of 2 are respectively composed of substantially the same shape (diameter), structure, and material as the unillustrated endoscope insertion part that undergoes sterilization treatment, and functions as an example of the pipe receiving part in the claims;...
Embodiment 2
[0099] Next, Example 2 of the sterilization test kit for an endoscope of the present invention will be described. The endoscope sterilizing test unit of Example 2 has a structure including a single tube accommodating portion for accommodating two tubes. Below, refer to Figure 3 to Figure 10 , to illustrate the second embodiment.
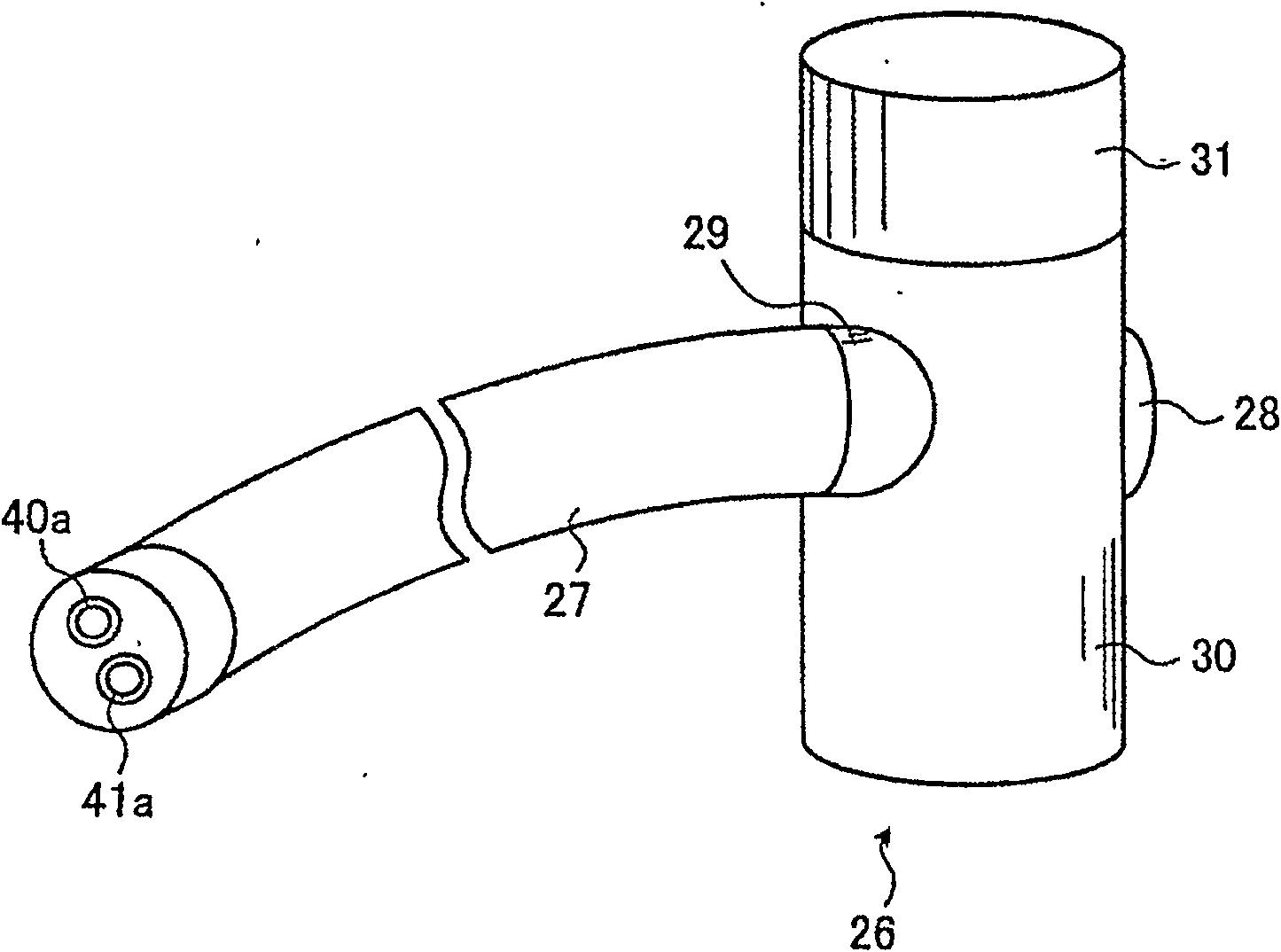
[0100] image 3 It is a schematic diagram showing the appearance of the endoscope sterilization test unit of Example 2. Such as image 3 As shown, the endoscope sterilization test assembly of the present embodiment 2 has the following parts: the outer shell 26 is used to accommodate the inner shell 33 (not shown in the figure) that contains the indicator. image 3 Expressed in); accommodated the first pipeline 38 and the second pipeline 39 (not in image 3 Indicated in the pipeline housing part 27 of); One-way valve 28, before introducing sterilizing gas, make the inner space of shell 26 and the inner space of pipeline housing part 27, that is,...
Deformed example 1
[0136] Next, Modification 1 of Embodiment 2 will be described. The endoscope sterilization test assembly of the modification 1 also has the following mechanism, which can detect the internal space of the outer casing 26 and the pipe receiving part 27 (in other words, the inner casing 33, the second Whether the surroundings of the first pipe 38 and the second pipe 39) maintain a substantially vacuum atmosphere.
[0137] Figure 11 It is a schematic diagram showing this mechanism and its surrounding structures. Such as Figure 11 As shown, on the housing case 55 as the structural element of the housing, in addition to the connecting portion with the upper cover 31, a through hole communicating with the external space is also formed. The endoscope sterilization test unit in Modification 1 has a structure in which the through hole is covered by bonding and fixing the flexible thin plate 56 in a substantially airtight manner.
[0138]The flexible thin plate 56 is used as a vacu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com