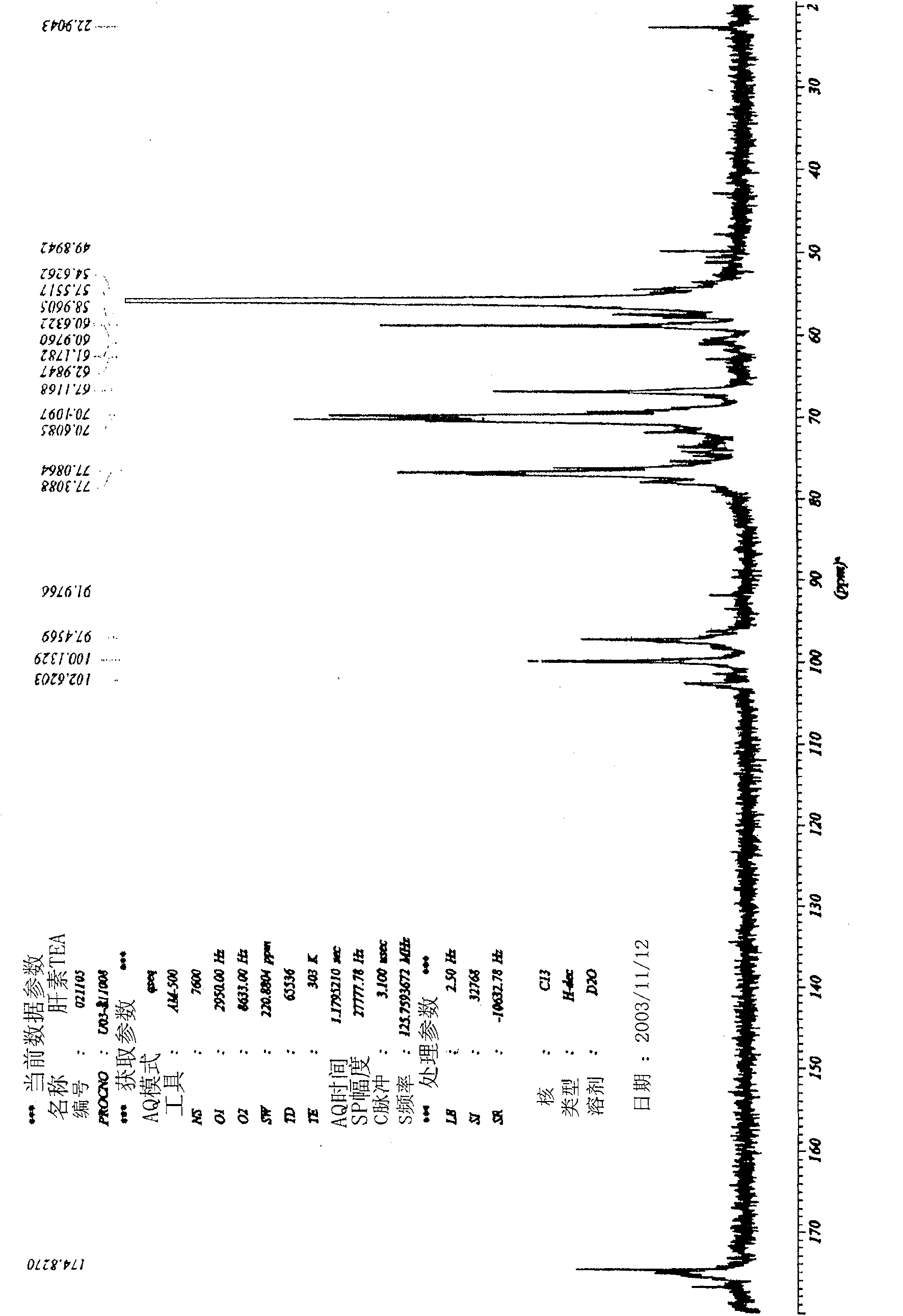
Low molecular weight heparin triethanolamine salt usable as local delivery antithrombotic treating agent, preparing process and use thereof
A low-molecular-weight heparin, ethanolamine salt technology, applied in the direction of organic active ingredients, medical preparations containing active ingredients, blood diseases, etc., can solve problems such as restricting the use of antithrombotic agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Depolymerization of heparin
[0037] 100 g of USP / EP grade heparin for injection was dissolved in sufficient distilled water to obtain 500 ml of a 20% solution.
[0038] The solution was placed in a 4-necked spherical bottle equipped with a stirrer, pH meter electrode, heating and reflux cooling device. The aqueous solution was heated to 94-96°C, followed by the addition of 1M hypochlorous acid solution (prepared by adjusting the pH of a pre-cooled 80 g / l sodium hypochlorite solution to 6 with hydrochloric acid) at 0 / 5°C. 290 mL of 1 M HClO solution was added over a 60 minute reaction time, while HCl or NaOH was added to the aqueous solution as needed to keep the pH stable at 5.0-6.0.
[0039] After the 60-minute reaction was over, 3% sodium chloride was added and 2 times the volume of ethanol was added with vigorous stirring to cause rapid precipitation of the reaction product, resulting in immediate termination of the depolymerization reaction.
[0040] Salt for...
Embodiment 2
[0058] Depolymerization of heparin
[0059] 100 grams of USP / EP grade heparin for injection was dissolved in sufficient distilled water to obtain 500 ml of a 20% (w / v) solution and transferred to a balloon provided with heating, stirring and reflux cooling.
[0060] will consist of 20 ml of 30% H 2 o 2 and 1 ml of 1.5% FeSO 4 ·7H 2 Reagents of composition O were heated to 80°C and added with stirring.
[0061] After heating and stirring for 40 minutes, the reaction was stopped immediately by adding 2% NaCl and precipitating the depolymerized polysaccharide by adding 2 volumes of ethanol.
[0062] The obtained precipitate was dissolved in a sufficient amount of distilled water to obtain a 10% solution, and the solution was applied to a 1 liter column capacity (φ5.2 cm × 50 cm height) containing ion exchange resin ( IR-120 (Rohm Haas) (phase H + ) column, flow rate 0.42 cm 3 / cm 2 × minutes. The acidic eluate was collected in an ice bath and its pH was adjusted to 5....
Embodiment 3
[0079] Lyophilization in the presence of mannitol: addressing the inherent hygroscopicity of LMWH-TEA
[0080] 18.6 g of LMWH-TEA obtained in Example 2 was dissolved in sufficient distilled water to obtain 125 ml of a 15% (weight to volume) solution. While stirring, 12.40 grams of USP grade mannitol was added, as in this example, mannitol is very soluble at room temperature when the initial solution concentration is not higher than 15%.
[0081] The solution was lyophilized to obtain a dry powder of the crystals with greatly reduced hygroscopicity, which was ready for grinding. The ground powder is fluid and does not agglomerate.
[0082] The following analysis table is provided for comparison:
[0083] LMWH-TEA LMWH-TEA / Mannitol
[0084] Anti-Xa activity 50 IU / mg 30 IU / mg
[0085] Anti-IIa activity 24 IU / mg 14 IU / mg
[0086] Sulfur (S) 6.8% 4.2%
[0087] S / COO - Ratio 2.1 2.1
[0088] Triethanolamine 50% 30%
[0089] Sodium (Na) 280ppm 170ppm
[0090...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap