Usage of eleutheroside with anti-inflammatory action or aglycone thereof
A technology of aglycone and inflammation, which is applied in the field of medical application of arachnid and its aglycone, which can solve the problems of lagging intervention methods and unsatisfactory curative effect of liver failure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
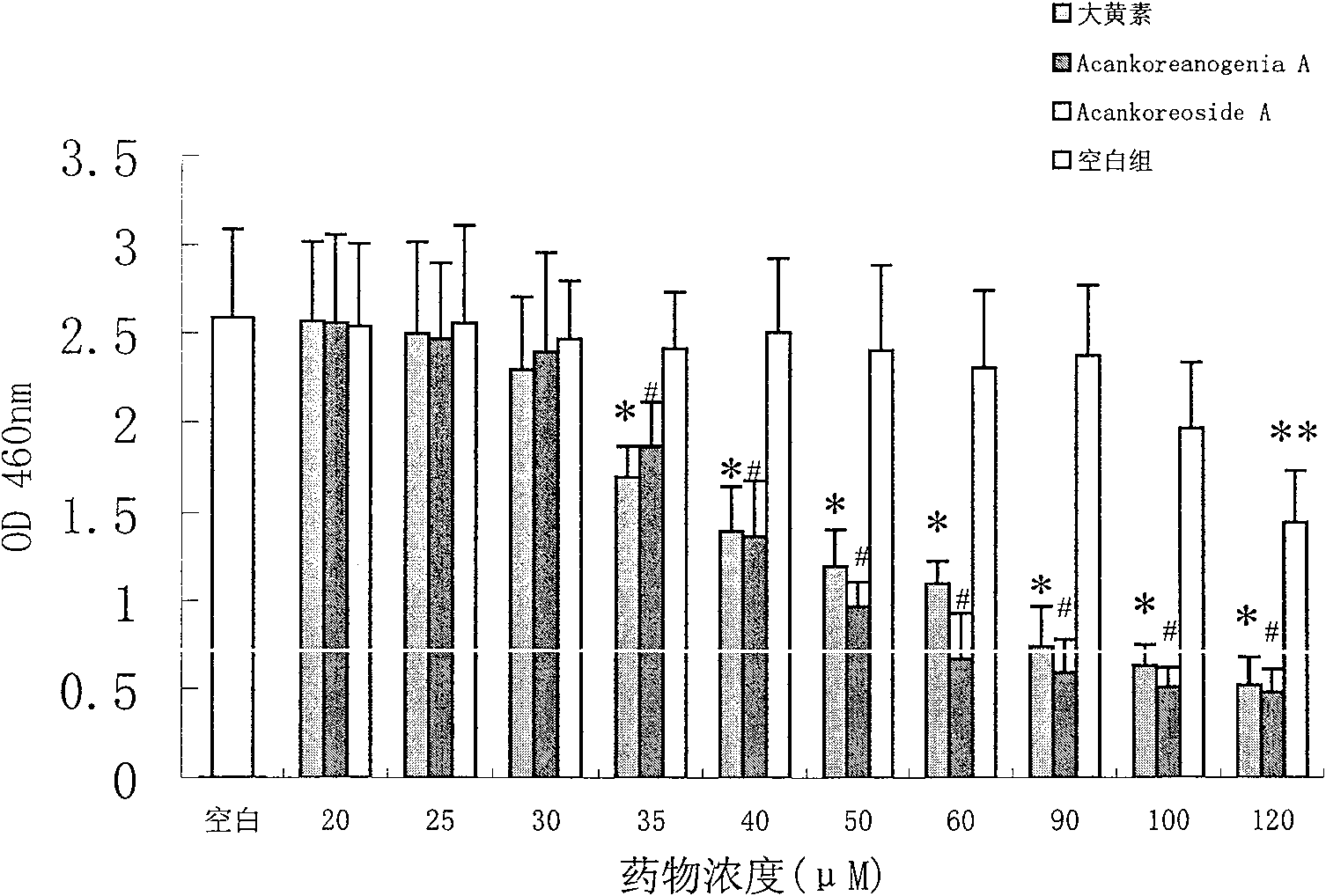
[0045] Example 1: Cytotoxicity of Target Drugs
[0046] EZ4U Cell Proliferation and Cytotoxicity Analysis Kit found that both formulas I and II have certain toxic effects on cells. Wherein, formula II is highly toxic to cells, and has an influence on the growth of RAW 264.7 cells when it is greater than 30 μM. The results are shown in figure 1 shown.
Embodiment 2
[0047] Example 2: Effects on RAW 264.7 TNF-α, IL-1β secretion
[0048] ELISA detection of RAW 264.7 medium supernatant revealed that both formulas I and II could reduce the secretion of TNF-α and IL-1β, and within a certain range, they were all in a dose-dependent manner. The specific results are shown in Tables 1-2 below.
[0049] Table 1 ELISA detection of TNF-α, IL-1β levels in Acankoreanogenia A intervention group RAW 264.7 medium supernatant (mean±SD)
[0050]
[0051] LPS - Indicates that no LPS was added to the culture medium, LPS + Indicates the addition of LPS with a final concentration of 100ng / ml in the culture medium. * p, ** P+ Group.
[0052] Table 2 ELISA detection of TNF-α and IL-1β levels in Acankoreoside A intervention group RAW 264.7 medium supernatant (mean±SD)
[0053]
[0054] LPS - Indicates that no LPS was added to the culture medium, LPS + Indicates the addition of LPS with a final concentration of 100ng / ml in the culture medium.
[0055...
Embodiment 3
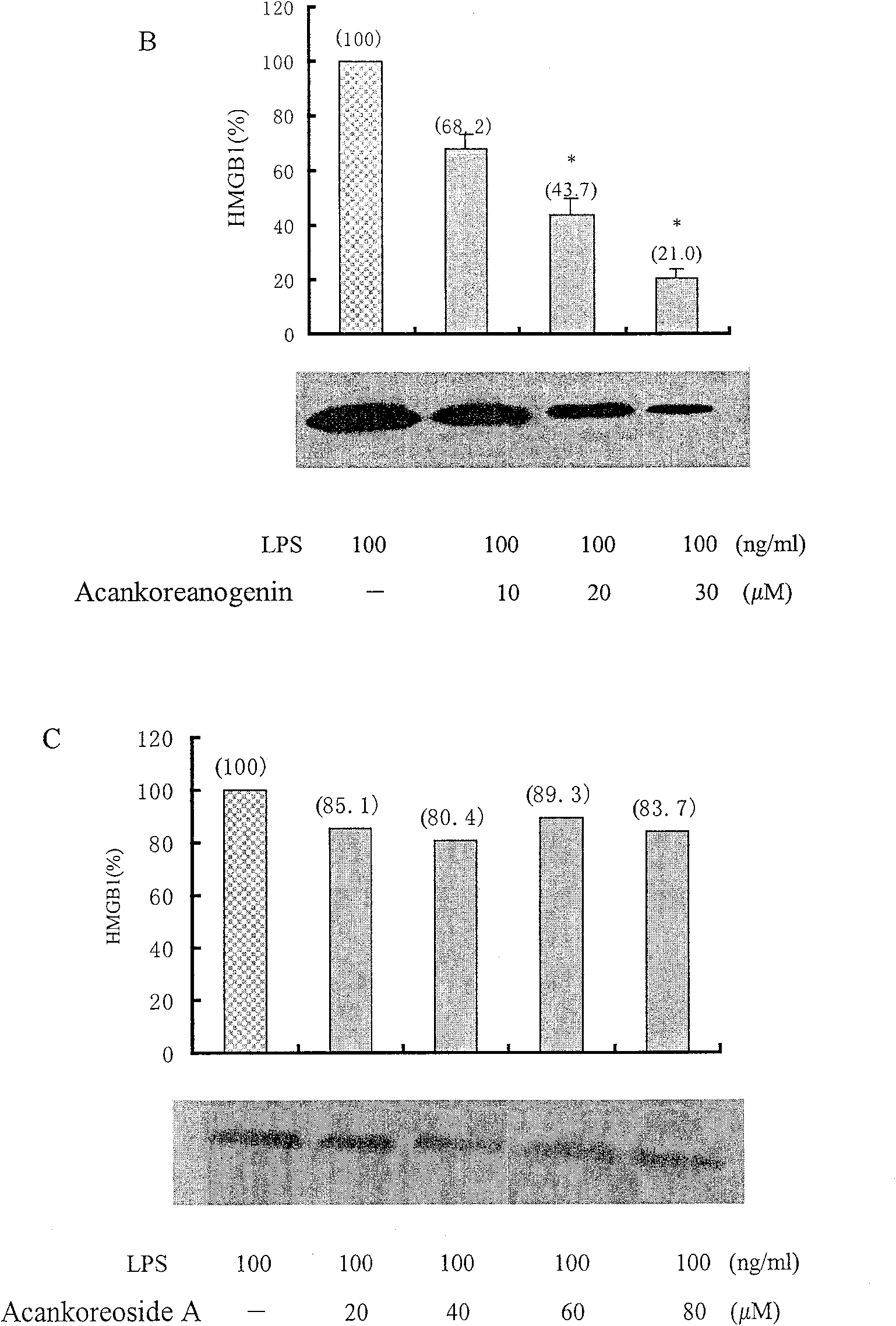
[0056] Example 3: Effects on RAW 264.7HMGB1 secretion
[0057] The results reflect that both formulas I and II can inhibit the secretion of HMGB1 in a dose-dependent manner within a concentration range of less than 30 μM. For specific results, see figure 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com