Trichina paramyosin gene and application thereof
A Trichinella spiralis, amino acid technology, applied in the fields of application, anti-animal/human immunoglobulin, gene therapy, etc., can solve the problems of complexity and diversity of Trichinella spiralis antigens, limited antigen acquisition, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Example 1 Cloning and sequence analysis of Trichinella spiralis paramyosin gene
[0093] 1. Preparation of screening serum (artificially immunized rabbit serum and artificially infected rabbit serum)
[0094] Adults of Trichinella spiralis Heilongjiang (International Standard Strain Code ISS533) isolated from pigs were collected, and the adult bodies were placed in a grinder and rapidly ground for 15 minutes under ice bath. Transfer the ground liquid into a 10ml centrifuge tube, and ultrasonicate (on ice) 3 times, 3 minutes each time, with a 1-minute rest in between. Repeated freezing and thawing several times, centrifuging and taking the supernatant to obtain the whole worm soluble antigen. Immunize Japanese big-eared white rabbits (1.6mg / rat), boost once a week, and boost 4 times to obtain artificially immune rabbit serum; experimental rabbits were fed with 4000 Trichinella muscle larvae, and artificially infected rabbit serum was obtained after 4 weeks. As determin...
Embodiment 2
[0165] Example 2 Preparation of Paramyosin Gene Recombinant Protein and Artificial Immunization Rat Serum
[0166] 1. Prokaryotic expression and purification of paramyosin gene
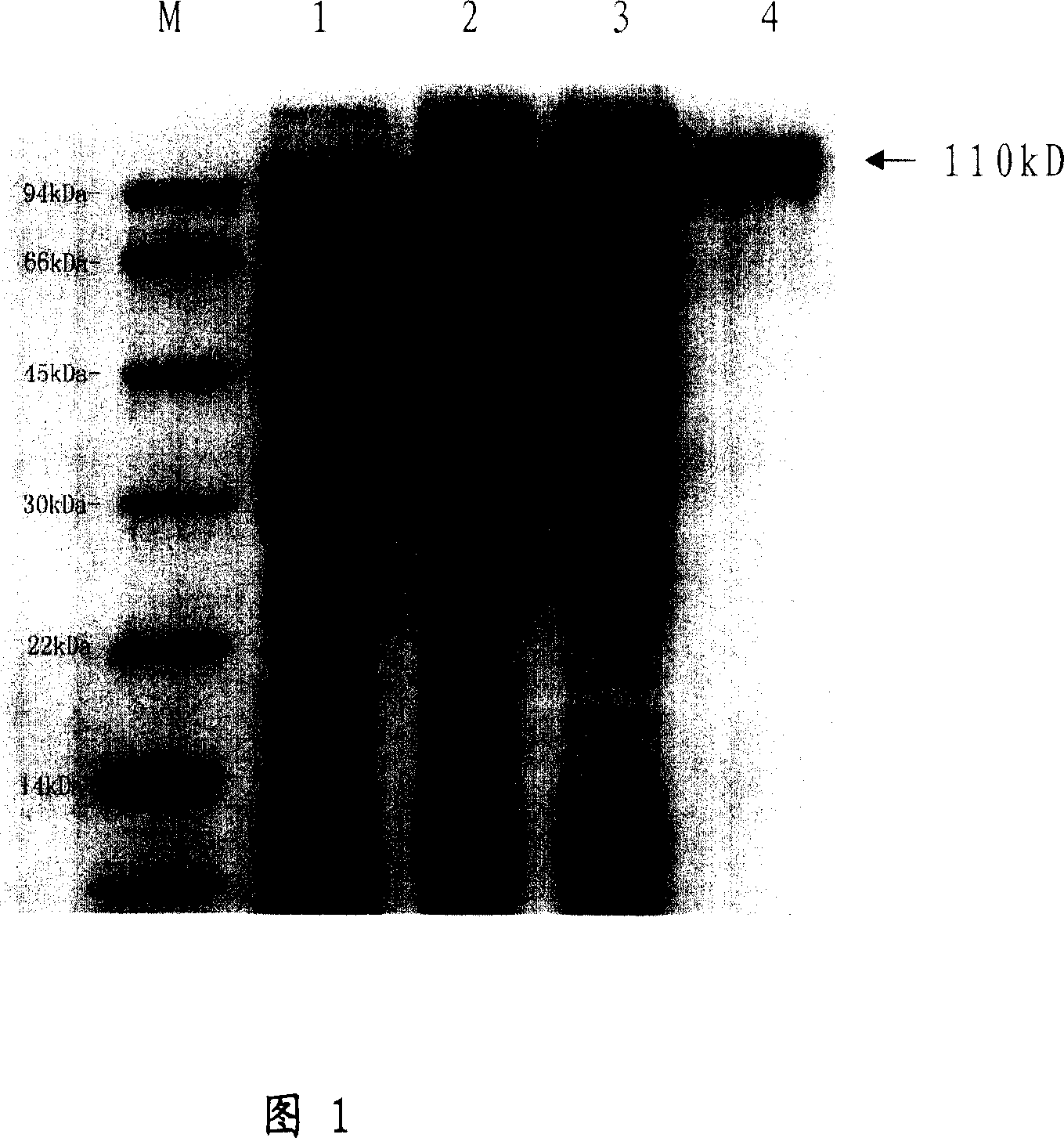
[0167] According to the Ts86 cDNA sequence, a pair of primers were designed, the upstream primer was 5'-CGGGATCCATGTCTCTGTATCGCAGTCCCAGT-3' (SEQ ID NO: 3) (32 nt in total, containing a BamH I restriction site), and the downstream primer was 5'-CGGAATTCATATTCATGTCCTTCTCCATCAC-3 '(SEQ ID NO: 4) (total 32nt, containing an EcoR I restriction site). The Ts86 cDNA clone obtained from the immune screening library was used as a template for PCR, and the product was subcloned into the prokaryotic expression vector PET-28a(+) (Novagen Company) after being digested with BamH I and EcoR I. The correct recombinant plasmid was transformed into Escherichia coli BL21 strain (Novagen Company), and induced by 1.0mM IPTG at 150 rpm at 37°C for 3 hours to obtain the recombinant protein of the paramyosin gene (885 amino ...
Embodiment 3
[0169] Embodiment 3 Immune characteristics of paramyosin gene recombinant protein
[0170] 1. Enzyme-linked immunosorbent assay (ELISA) detection
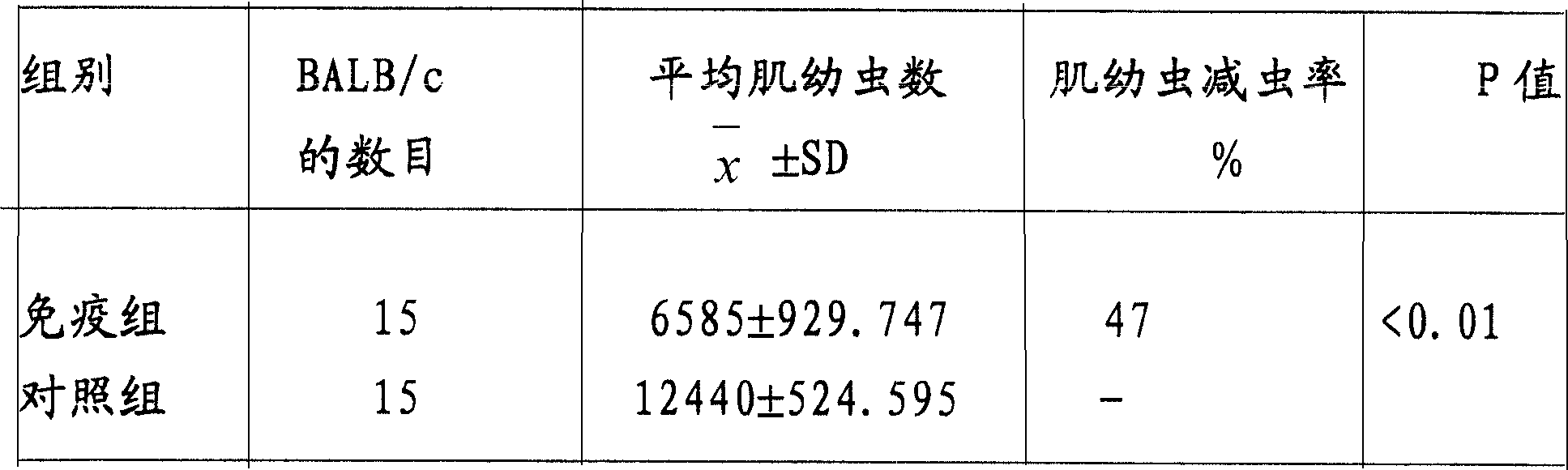
[0171] 1 μg of paramyosin gene recombinant protein (prepared in Example 2) was used as an antigen to coat a 96-well microtiter plate, the primary antibody was the serum to be tested, and the secondary antibody was the corresponding horseradish peroxidase-labeled (goat anti-human, goat Anti-rabbit or goat anti-pig) secondary antibody, normal human serum, normal rabbit serum, and normal pig serum were used as negative controls. According to the OD value of the serum sample to be tested is greater than 2.1 times of the negative control, it is a positive judgment result. After ELISA detection, the positive rate of trichinellosis patient serum (23 copies) was 86.96%, trichinellosis rabbit serum (2 copies) and trichinellosis pig serum (4 copies) were all positive, indicating that the recombination of the paramyosin gene The protein has...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com