Capsule stable against mastication
A technology of capsules and capsule shells, which can be used in the fields of capsule delivery, inactive components of polymer compounds, and nervous system diseases, etc., and can solve problems such as no specific disclosure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
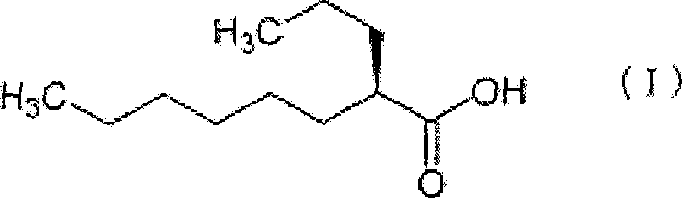
[0149] Embodiment 1: the preparation of the soft capsule containing (2R)-2-propyl octanoic acid (300mg)
Embodiment 1-1
[0151] Composition of capsule shell Bovine gelatin: glycerol = 100:30
[0152] Bovine gelatin (20 kg) and concentrated glycerin (6 kg) were mixed at 70°C in the presence of purified water (20 kg) to obtain a homogeneous solution. This solution and (2R)-2-propyl octanoic acid (0.9kg) are dropped into soft capsule filling machine (rotary soft capsule forming machine H-1 type; Kamata), obtain filling (2R)-2-propyl octanoic acid Softgels in raw balls. By drum-drying the obtained green pellets (24°C, 3 hours) and rack drying (29°C, 15-45 hours) in sequence to obtain 300 mg of (2R)-2-propyloctanoic acid in each capsule Softgels (2100 Capsules). Carry out the same operation 6 times further, obtain altogether 7 batches of soft capsules. The rack drying time for each batch was: 27 hours for batches #1 to #5, 15 hours for batch #6, and 45 hours for batch #7.
Embodiment 1-2
[0154] Composition of capsule shell Porcine gelatin: glycerol = 100:30
[0155] Porcine gelatin (20 kg) and concentrated glycerol (6 kg) were mixed at 75°C in the presence of purified water (16 kg) to obtain a homogeneous solution. The solution and (2R)-2-propyl octanoic acid (1.8kg) were put into a soft capsule filling machine (Rotary (ロ-タリ-) type soft capsule molding machine H-1 type; Kamata) to obtain filling (2R) -2-Propyl octanoic acid in raw pellets of soft capsules. The obtained green pellets were subjected to drum drying (23.5°C, 3 hours) and rack drying (29°C, 27 hours) in sequence to obtain soft-shelled (2R)-2-propyloctanoic acid containing 300 mg per capsule. capsules (5700 capsules).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Strength | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com