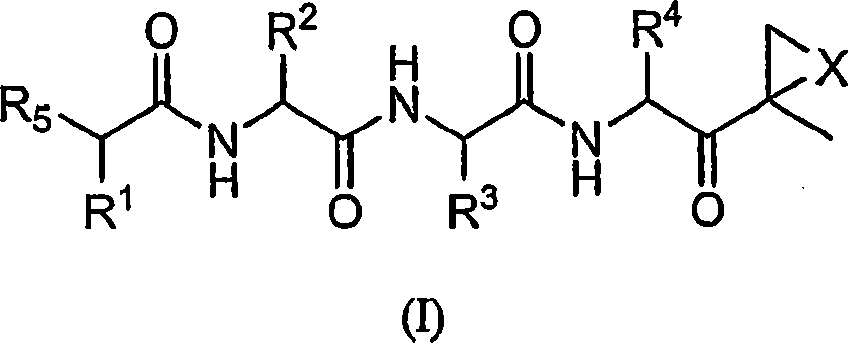
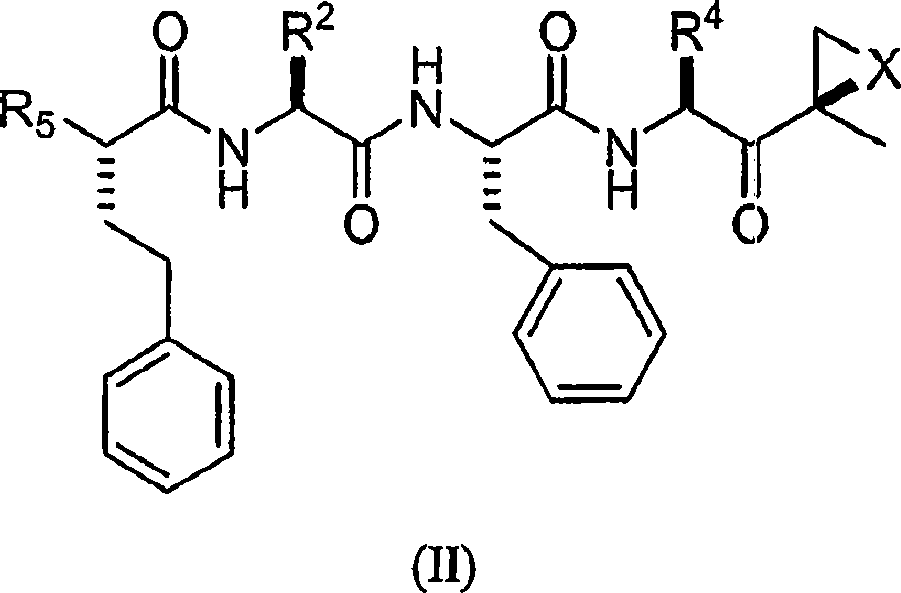
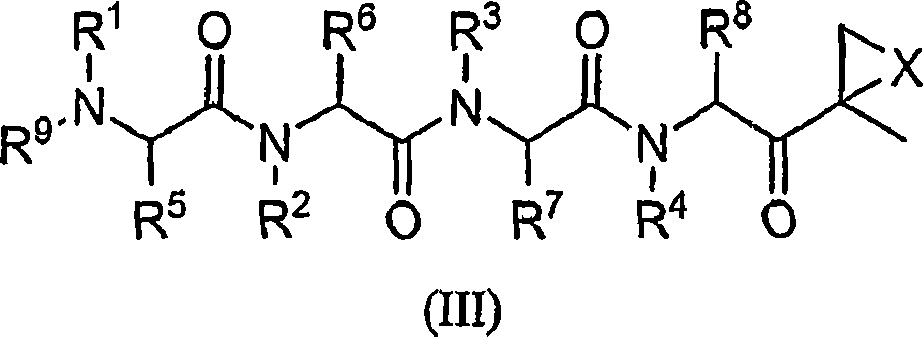
Compounds for proteasome enzyme inhibition
A compound, alkyl technology for enzyme inhibition-based therapeutics addressing issues of lack of specificity, stability or potency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0222] Scenario 1: Example 1 Synthesis
[0223]
[0224] (B) Synthesis
[0225]HOBT (10.81 g, 80.0 mmol) and DIEA (200.0 mmol, 25.85 g , 35mL). The mixture was cooled to 0°C in an ice bath, and BOP (80.0 mmol, 35.38 g) was added in portions over 5 minutes. The reaction solution was placed under an argon atmosphere and stirred overnight. The reaction was diluted with brine (1000 mL), and extracted with ethyl acetate (5 x 200 mL). The combined organic layers were washed with water (10 x 100 mL) and brine (2 x 150 mL), dried over magnesium sulfate. Magnesium sulfate was removed by filtration and volatiles were removed under reduced pressure to afford (A) (18.17g). BocNHLeuPheOMe (45.86 mmol, 18.0 g) was added to 50 mL of 80% TFA / DCM solution cooled at 0°C. The solution was stirred and allowed to warm to room temperature over 2 hours. The volatiles were removed under reduced pressure to give an oil. To this oil was added BocNHhPhe (45.86 mmol, 12.81 g), DMF (500 mL), H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com