Compounds for proteasome enzyme inhibition
A technology for compounds, drugs, applied in the therapeutic field based on enzyme inhibition, which can solve problems such as lack of specificity, stability or efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
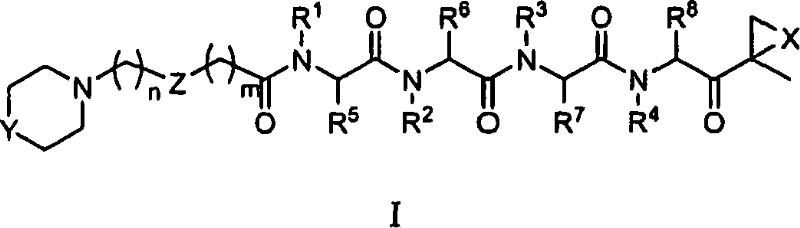
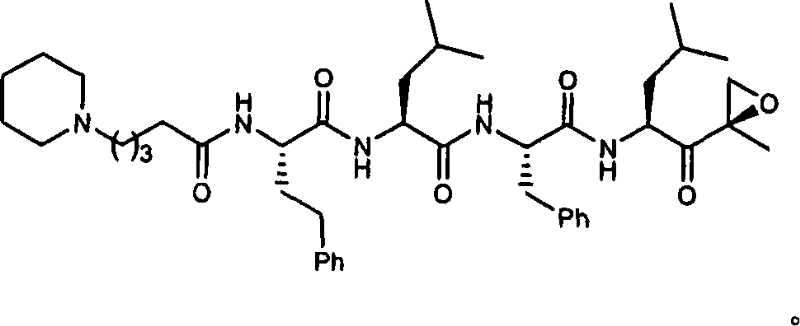
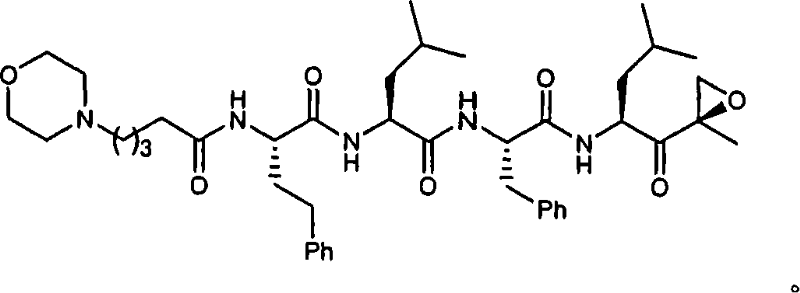
[0015] The present invention relates to compounds useful as enzyme inhibitors. These compounds are generally useful for inhibiting enzymes with a nucleophilic group at the N-terminus. For example, the activity of enzymes or enzyme subunits containing an N-terminal amino acid having a nucleophilic group in the side chain, such as threonine, serine, or cysteine, can be successfully inhibited by the enzyme inhibitors described herein. The activity of enzymes or enzyme subunits having a non-amino acid nucleophilic group at the N-terminus, such as a protecting group or a sugar group, can also be successfully inhibited by the enzyme inhibitors described herein.
[0016] While not wishing to be bound by any particular theory, it is believed that the N-terminal nucleophilic group of Ntn forms a covalent adduct with the epoxy functionality of the enzyme inhibitors described herein. For example, in the β5 / Pre2 subunit of the 20S proteasome, it is generally believed that the N-terminal ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com