Process for the manufacture of chlorodifluoromethane
A production method and condenser technology, applied in the field of preparation of chlorodifluoromethane
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
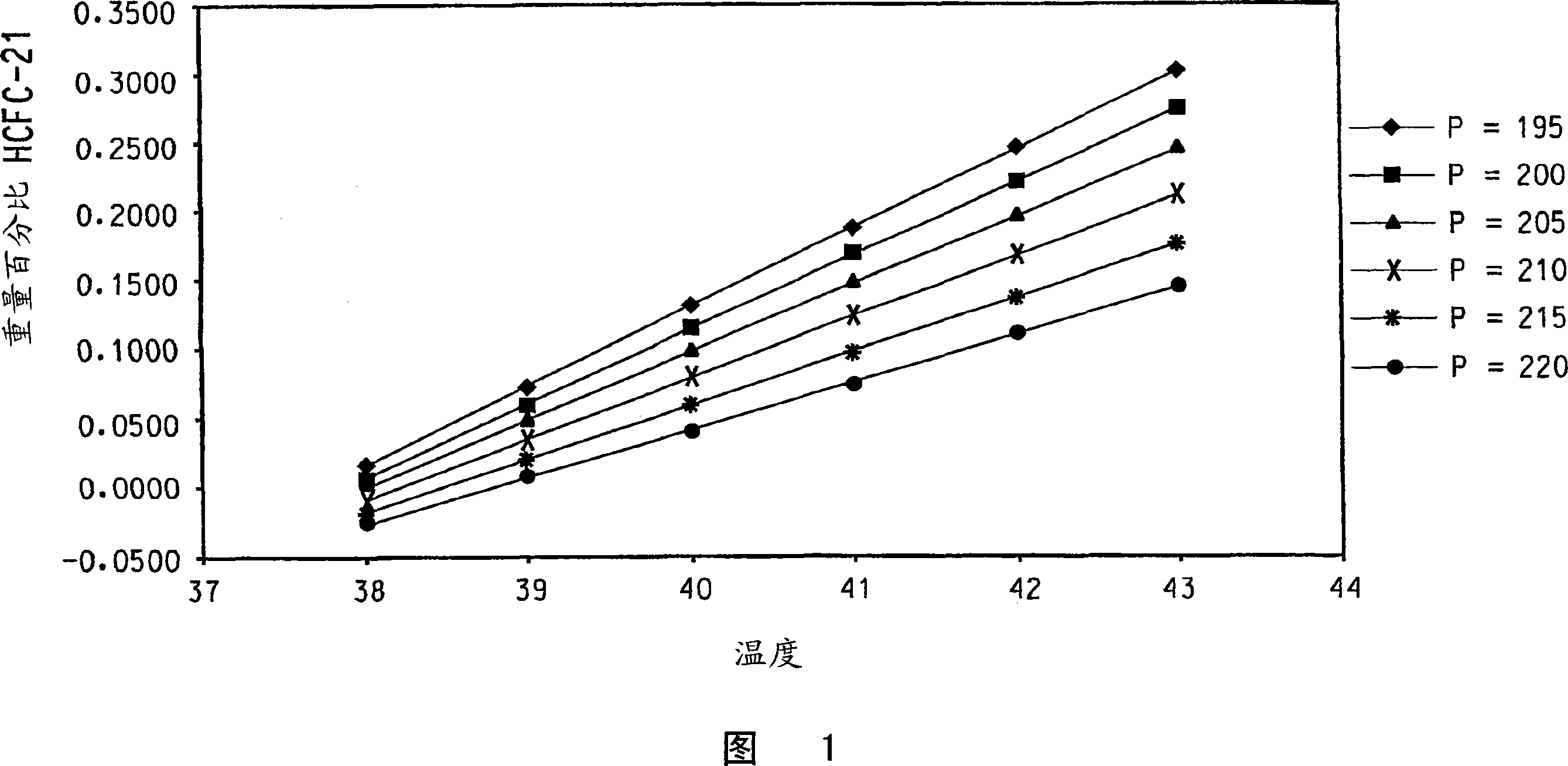
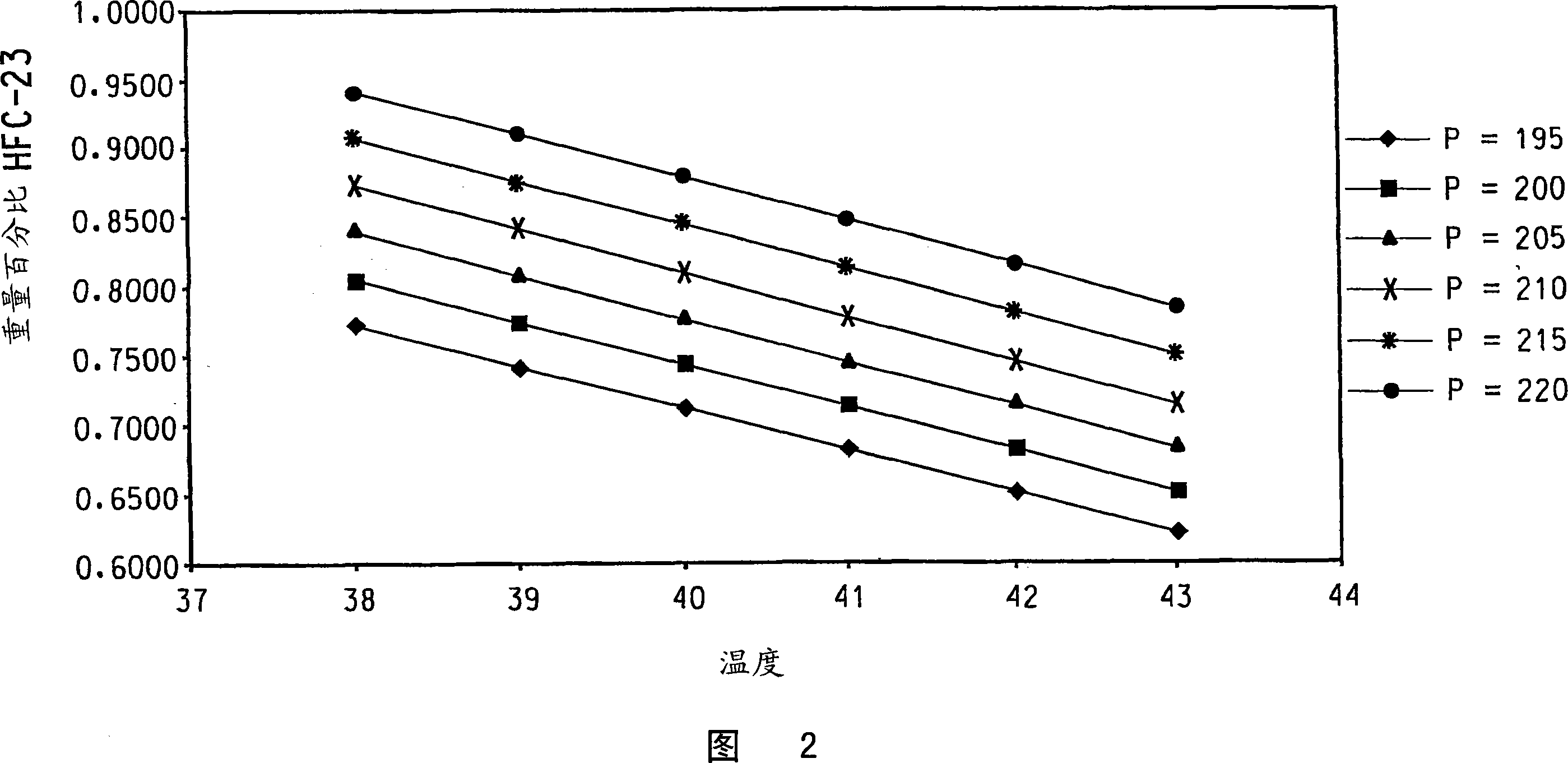
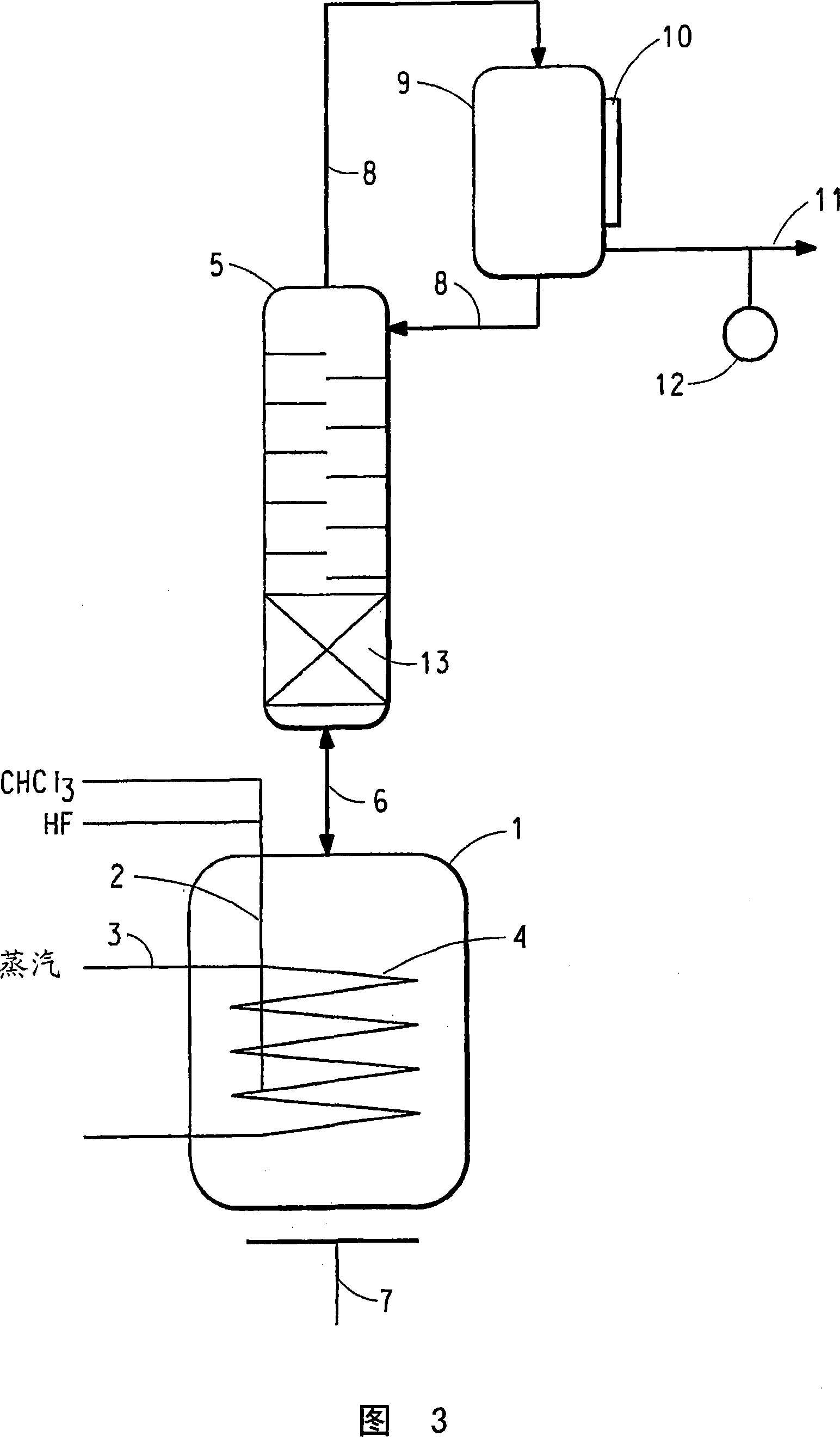
[0070]The reaction apparatus and procedure of Comparative Example 1 were used in this example with the following changes. The reactor liquid phase is maintained at the maximum mass that does not cause entrainment and / or flooding of the reactor liquid phase into the reflux column 5 . A brine cooling medium is circulated through the cooling jacket 10 of the condenser 9 and is used to set the reflux ratio of the condenser 9 to be substantially constant during steady state operation of the reactor so that part of the reflux column vapor effluent condenses and forms 2 of the condenser liquid effluent, and have reduced or substantially no CHCl 2 F and CHF 3 containing CHClF 2 and HCl in the condenser vapor effluent. The reflux ratio averaged 1.60 (min 1.41, max 1.79). The temperature of a point within the lower 1 / 3 of the theoretical stage of the reflux column is 41.5°C on average (minimum 37.9°C, maximum 43.4°C). The amount of pentavalent antimony catalyst present in the liqui...
Embodiment 2
[0077] The reaction apparatus and procedure of Example 1 were used in this example with the following changes. The reflux column of Example 1 was replaced with a cylindrical column of the same dimensions, however, the bottom 1 / 3 of the length of the reflux column contained a packed bed of stainless steel #25 IMTP packed packing (from Norton Chemical Process Products Co., Ohio, USA), The top 2 / 3 of the reflux column contained 18 trays and had no packing. The average reflux ratio was 1.59 (minimum 1.53, maximum 1.99). The temperature of a point within the lower 1 / 3 of the theoretical stage of the reflux column is 45.9°C on average (minimum 36.5°C, maximum 48.7°C). The amount of pentavalent antimony catalyst present in the liquid phase of the reactor averaged 28.4 wt% (minimum 27.6 wt%, maximum 29.2 wt%). CHCl via line 2 to the reactor 3 The / HF feed ratio averaged 2.78 (min 1.71, max 2.95). Reactor pressure measured at the upper end of the reflux column averaged 199.8 psig (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com