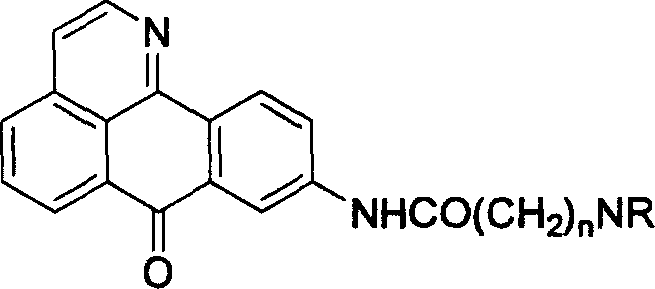
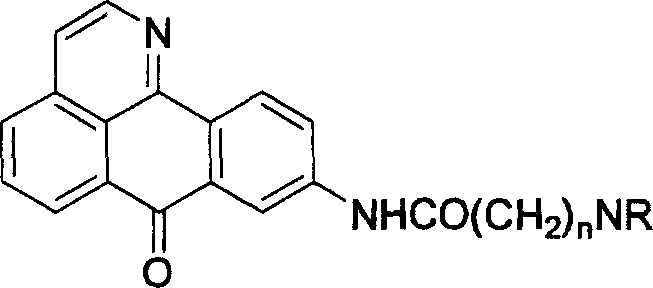
9-aminoalkylamido-1-azabenznthrone derivative and its synthesis and application
A technology of aminoalkanoyl amido and benzanthrone, which is applied in the field of 9-aminoalkanoyl amido-1-azabenzoanthrone derivatives, can solve the problem of increased activity of butyrylcholinesterase, etc. Achieve the effects of reducing drug side effects, enhancing drug efficacy, and significantly inhibiting selectivity and specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Synthesis of 9-(2-chloroacetamido)-1-azabenzoanthrone
[0023] 1.0g (4mm) of 9-amino-1-azabenzanthrone was refluxed in 10ml of chloroacetyl chloride for 3-4 hours, cooled, filtered, washed with ether, and the filtrate was recrystallized with DMF-ethanol to obtain yellow needles (compound 1) 1.1 g (about 85% yield). 1 H NMR (DMSO, 300MHz): δ4.35(s, 2H), 7.98(d, 1H, J=5.6Hz), 8.01-8.09(m, 2H), 8.41(d, 1H, J=8.2Hz), 8.51(d, 1H, J=2.2Hz), 8.54(d, 1H, J=7.2Hz), 8.73-8, 78(m, 2H), 10.76(s, 1H-CONH); ESI-MS m / z : 324[M+H] + .
[0024] The structural formula of the synthesized compound 1 is as follows:
[0025]
Embodiment 2
[0026] Example 2: Synthesis of 9-(3-chloropropionamido)-1-azabenzanthrone
[0027] 1.0g (4mm) of 9-amino-1-azabenzanthrone was refluxed in 10ml of chloropropionyl chloride for 3-4 hours, cooled, filtered, washed with ether, and the filtrate was recrystallized with DMF-ethanol to obtain yellow needles (compound 2) 1.0 g (about 74% yield). 1 H NMR (DMSO, 300 MHz): δ2.92 (t, 2H, J = 6.2Hz), 3.93 (t, 2H, J = 6.2Hz), 7.98 (d, 1H, J = 5.6Hz), 8.01-8.11 (m, 2H), 8.42(d, 1H, J=8.2Hz), 8.54-8.56(m, 2H), 8.73-8.78(m, 2H), 10.55(s, 1H-CONH); ESI-MS m / z: 338 [M+H] + .
[0028] The synthetic compound 2 structural formula is as follows:
[0029]
Embodiment 3
[0030] Example 3: Synthesis of 9-(4-chlorobutanylamino)-1-azabenzanthrone
[0031] 1.0g (4mm) of 9-amino-1-azabenzanthrone was refluxed in 10ml of chlorobutyryl chloride for 2.5 hours, cooled, filtered, washed with ether, and the filtrate was recrystallized with DMF-ethanol to obtain yellow needle-like crystals (Compound 3) 1.0 g (about 74% yield). 1 H NMR (DMSO, 300MHz): δ2.09(m, 2H), 2.58(t, 2H, J=7.3Hz), 3.74(t, 2H, J=6.5Hz), 7.98(d, 1H, J=5.6 Hz), 8.01-8.10(m, 2H), 8.41(d, 1H, J=8.1Hz), 8.54-8.56(m, 2H), 8.72-8.78(m, 2H), 10.46(s, 1H-CONH) ;ESI-MS m / z: 352[M+H] + .
[0032] The synthetic compound 3 structural formula is as follows:
[0033]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com