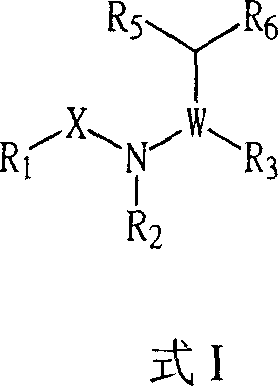
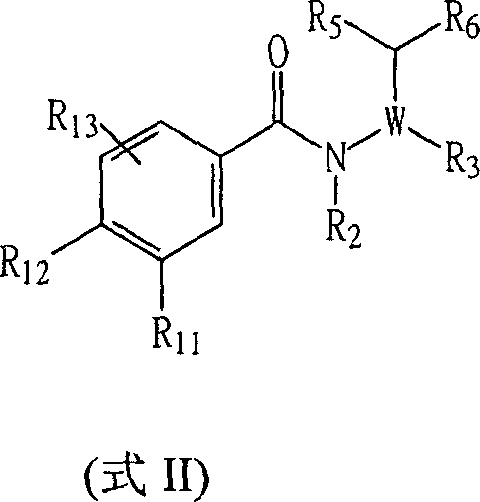
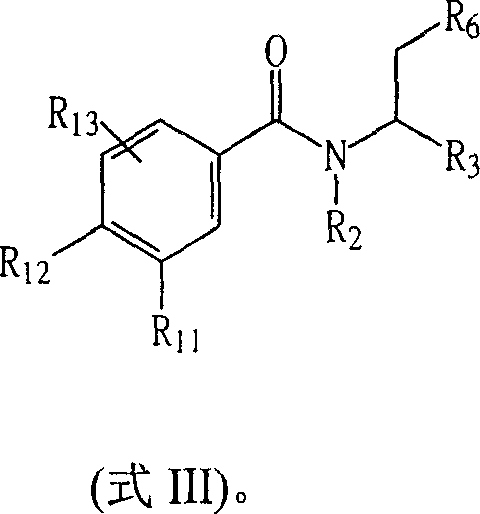
Certain chemical entities, compositions, and methods
A chemical and compound technology, applied in the field of chemicals, can solve the problems of mitotic spindle malformation, cell cycle arrest, obstacles, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0629]
[0630] To a solution of 4-isopropoxybenzoic acid (25 g, 140 mmol) in DMF (150 mL) was added NCS (24 g, 182 mmol). The reaction mixture was stirred overnight. h 2 O (500 mL) was added to the reaction mixture. The precipitate was collected and washed with water and dried under vacuum to yield 2 (26.4 g, 88%) as a white solid which was used in the next step without further purification. LRMS (M+H + ) m / z 213.0.
[0631] To a solution of 2 (20 g, 93 mmol) in DCM was added pentafluorotrifluoroacetate (20 mL, 112 mmol) and triethylamine (17 mL, 112 mmol) at 0°C. The solution was concentrated and the mixture was purified by flash column chromatography (100% DCM) to yield 3 (35 g, total) as a white solid. LRMS (M+H + ) m / z 586.3.
[0632] To a solution of 3 in DMF (2.0 molar), amino acid (1.2 equiv) and N,N-diisopropylethylamine (3 equiv) were added. The reaction was monitored by LC / MS. Upon completion, methylamine (2 M in THF, 1.5 equiv) and HBTU (1.5 equiv) were...
Embodiment 2
[0634]
[0635]To a solution of H-Phe-(4-Br)-OH (2, 2.5 g, 10 mmol) in DMF (20 mL) was added 3 (4.7 g, 12 mmol) and diisopropyl Ethylamine (5.4 mL, 30 mmol). The reaction mixture was monitored by LC / MS. Upon completion, methylamine (2M in THF, 7.7 mL, 15 mmol) and HBTU (5.8 mL, 15 mmol) were added to the reaction solution. The reaction mixture was stirred for 2 days. The mixture was filtered, and the filtrate was analyzed by RP-HPLC using acetonitrile and H 2 The O mixture was purified to yield 4 (2.3 g, 50%). LRMS (M+H + ) m / z 455.0.
[0636] To a suspension of 4 (71 mg, 0.16 mmol) in dioxane (1 mL), piperazine (16 mg, 0.19 mg, palladium(II) acetate (4 mg, 0.016 mmol), dicyclohexyl Phosphino-2'-(N,N'-dimethylamino)-biphenyl (6 mg, 0.016 mmol) and cesium carbonate (104 mg, 0.32 mmol).The resulting mixture was stirred at 110°C for 36 hours The reaction mixture was diluted with EtOAc. The organic layer was diluted with saturated NaHCO 3 (20 ml) and brine washing, in N...
Embodiment 3
[0638]
[0639] For H-Tyr-NH in DMF (5 ml) 2 HCl (2,830 mg, 3.8 mmol) solution, 3 (1.8 g, 4.5 mmol) and diisopropylethylamine (3.4 mL, 19 mmol) were added at room temperature. The reaction was stirred for 20 hours and filtered after adding water. The white precipitate was recrystallized from DCM and methanol to yield 4 (1.120 g, 78%) as white crystals. LRMS (M+H + ) m / z 377.1.
[0640] To a solution of 4 (50 mg, 0.13 mmol) in DMF (1 mL) was added (s)-(+)-3-bromo-2-methyl-1-propanol (0.083 mL, 0.8 mmol) and potassium carbonate (110 mg, 0.8 mmol). The resulting mixture was stirred at 0°C for 15 hours. The mixture was filtered, and the filtrate was analyzed by RP-HPLC using acetonitrile and H 2 The O mixture was purified to yield 1b (30 mg, 51%). LRMS (M+H + ) m / z 499.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com