Non-peptide bradykinin antagonists and pharmaceutical compositions therefrom
A compound and mixture technology, applied in the field of non-peptide compounds, can solve problems such as destruction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
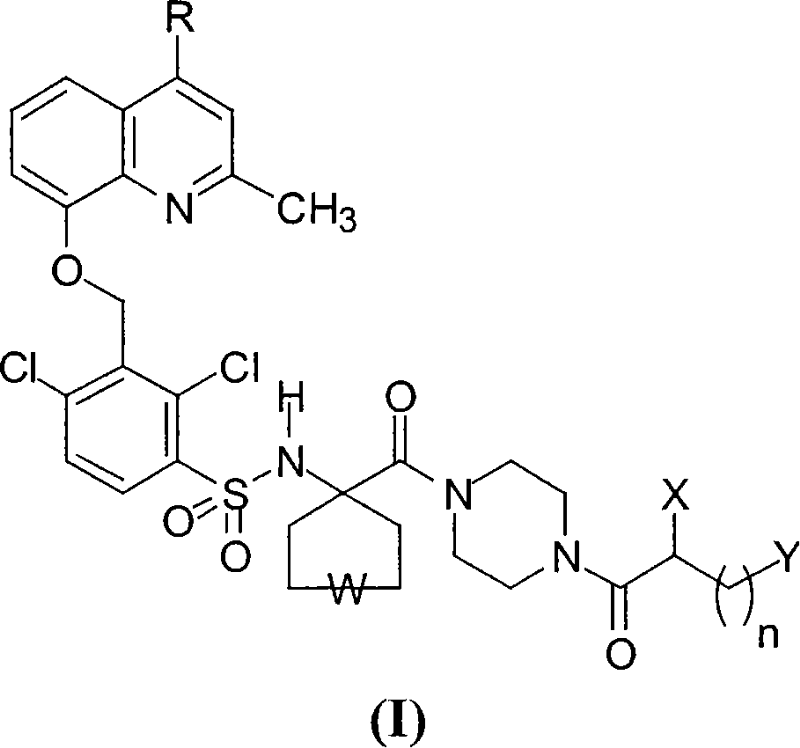
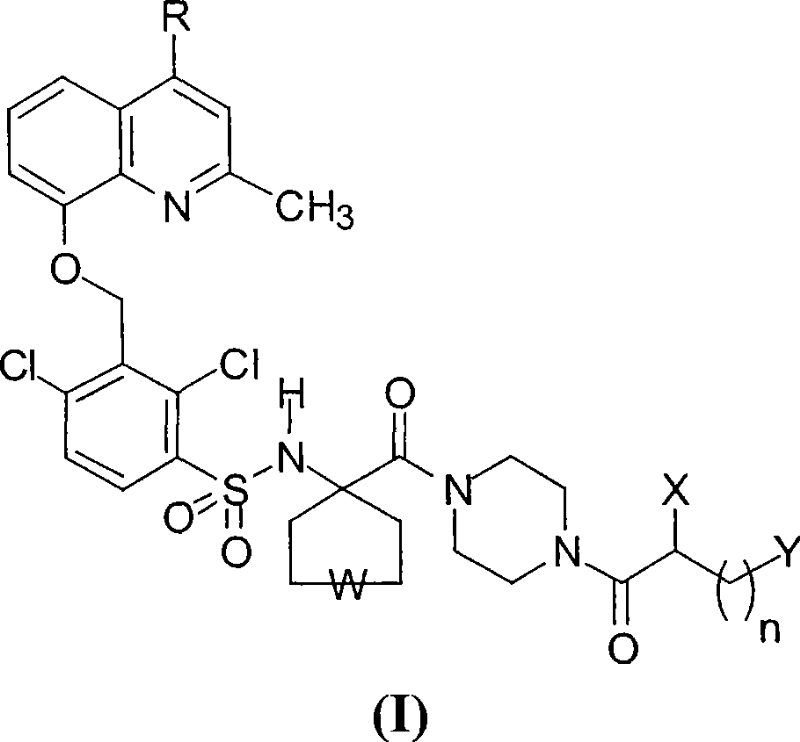
[0046] (4-(S)-amino-5-(4-{4-[2,4-dichloro-3-(2,4-dimethyl-quinolin-8-yloxymethyl)-benzenesulfonate Acylamino]tetrahydropyran-4-carbonyl}piperazin-1-yl)-5-oxo-pentyl)trimethyl-ammonium chloride, dihydrochloride
[0047] (compound of general formula I, wherein R=CH 3 , W=-O-, X=NH 2 , n=3, Y=N(CH 3 ) 3 + Cl - ).
[0048] The compound is synthesized according to the synthetic route illustrated in Scheme 2
[0049]
[0050] Example 1
[0051] Flowchart 2
[0052] General method: Analytical HPLC: Flow rate: 1 mL / min; Mobile phase: A - 0.1% trifluoroacetic acid in water, B - 0.1% trifluoroacetic acid in acetonitrile; Column: Zorbax Eclipse XDBC8, 5 microns, 150 x 4.6 mm.
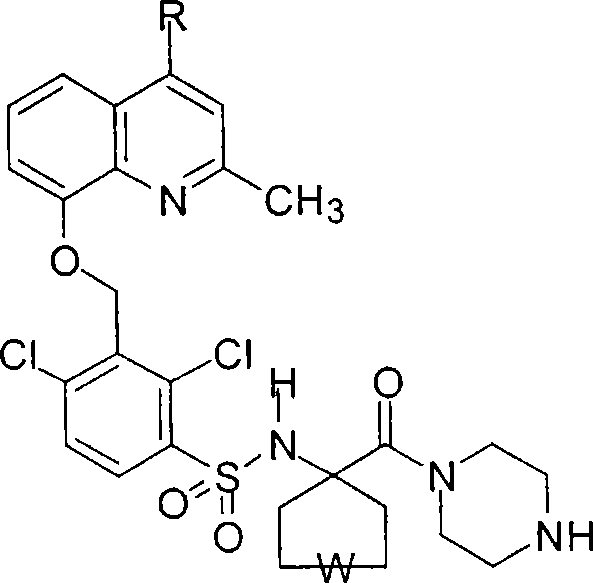
[0053] Intermediate (2) 2,4-dichloro-3-bromomethyl-benzenesulfonyl chloride
[0054] Under magnetic stirring at room temperature, 4.8 mL of 2,6-dichlorotoluene was added dropwise to 10 mL of chlorosulfonic acid over 2 hours. After the addition was complete,...
Embodiment 2
[0096] (4-(S)-amino-5-(4-(4-(2,4-dichloro-3-(2-methyl-quinolin-8-yloxymethyl)-benzenesulfonylamino) Tetrahydropyran-4-carbonyl)-piperazin-1-yl-)5-oxo-pentyl)-trimethylammonium chloride, hydrochloride
[0097] 1 H NMR (DMSO-d 6 )δ: 8.90 (1H, s), 8.47-8.34 (4H, m), 8.02 (1H, d), 7.81 (1H, d), 7.73-7.37 (4H, m), 5.62 (2H, s), 4.57 -4.45(1H,m), 4.01-3.56(5H,m), 3.43-3.18(7H,m), 3.06(9H,s), 2.78-2.61(4H,m), 2.89(1H,s), 1.97 -1.60 (9H, m). HPLC: retention time = 9.26 min. MS: [M] + 749.
Embodiment 3
[0099] [5-(4-{4-[2,4-dichloro-3-(2,4-dimethyl-quinolin-8-yloxymethyl)-benzenesulfonylamino]tetrahydropyran- 4-Carbonyl}piperazin-1-yl)-5-oxo-pentyl]-trimethylammonium trifluoroacetate.
[0100] 1 H-NMR (DMSO-d 6 ): δ (ppm) 1.53 (s, 2H, m); 1.69 (m, 4H); 1.90 (m, 2H); 2.45 (t, 2H); 2.78 (m, 6H); 3.04 (9H, s); 3.23-3.57 (7H, m); 5.68 (2H, s); 7.38-8.18 (5H, m); 8.04 (1H, d, J=8.42Hz); 8.82 (1H, s). HPLC: retention time = 5.65 minutes. MS: [M] + 748.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com