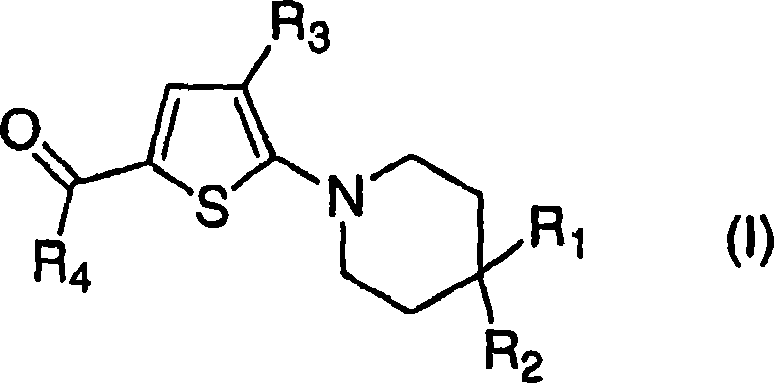
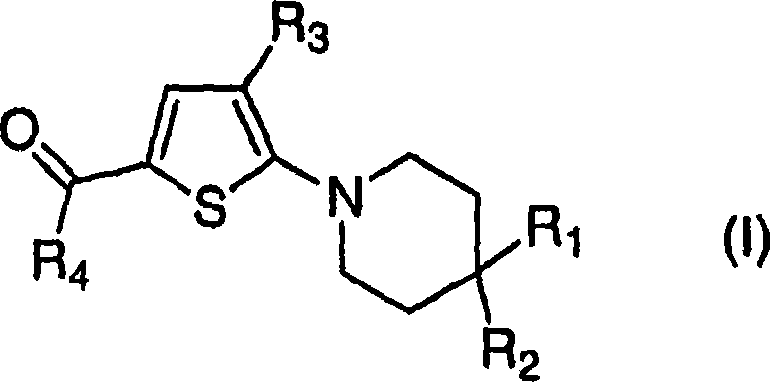
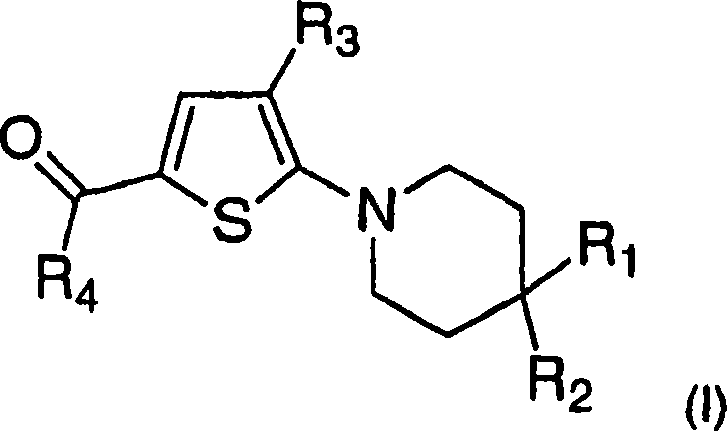
Trisubstituted thiophenes as progesterone receptor modulators
A compound and substituent technology, applied in the field of new tri-substituted thiophene derivatives, can solve problems such as side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] A, 1-(1,4-dioxa-8-aza-spiro[4.5]dec-8-yl)-2-pyridin-4-yl-ethylthione
[0114]
[0115] 1-Pyridin-4-yl-ethanone (12.1g, 0.1mol), sulfur (3.36g, 0.105mol) and 1,4-dioxa-8-aza-spiro[4.5]decane were mixed with p-toluene The sulfonic acid (0.50 g, 2.8 mmol) was mixed and heated to 120°C for 3 hours. The slurry was poured into methanol (50 mL). A pale yellow solid precipitated out. The solid precipitate was filtered and washed with another 20 mL of methanol. The solid was dried to give the product (24 g, 86.3%).
[0116] 1 H NMR (CD 3 OD) δ8.41(m, 2H), 7.42(m, 2H), 4.35(m, 4H), 3.91(m, 4H), 3.78(m, 2H), 1.80(m, 2H), 1.48(m, 2H). MS (m / z): 279 (MH + ).
[0117] B, 1-(1,4-dioxa-8-aza-spiro[4.5]dec-8-yl)-3-morpholin-4-yl-2-pyridine-4- yl-propenthione
[0118]
[0119] 1-(1,4-dioxa-8-aza-spiro[4.5]dec-8-yl)-2-pyridin-4-yl-ethylthione (20g, 72mmol), triethyl orthoformate (21.3g, 144mol), morpholine (48g, 55mmol) were stirred at 125°C for 4 hours. The solvent...
Embodiment 2
[0127] [5-(1,4-Dioxa-8-aza-spiro[4.5]dec-8-yl)-4-pyridin-4-yl-thiophen-2-yl]-(4- Fluoro-phenyl)-methanone
[0128]
[0129] With 1-(1,4-dioxa-8-aza-spiro[4.5]dec-8-yl)-3-morpholin-4-yl-2-pyridin-4-yl-propenthione and 2 Starting from -bromo-1-(4-fluoro-phenyl)-ethanone, the title product was prepared in 33% yield as described in Example 1C.
[0130] 1 H NMR (CDCl 3 )δ8.61 (dd, J=1.5 and 4.6Hz, 2H), 7.86-7.83 (m, 2H), 7.53-7.50 (m, 3H), 7.20-7.15 (m, 2H), 3.98 (s, 4H) , 3.23(t, J=5.7Hz, 4H), 1.83(t, J=5.7Hz, 4H); MS(m / z): 425(MH + ).
Embodiment 3
[0132] (4-fluoro-phenyl)-[5-(1,4-dioxa-8-aza-spiro[4.5]dec-8-yl)-4-pyridin-4-yl- Thiophen-2-yl]-methanone
[0133]
[0134] With 1-(1,4-dioxa-8-aza-spiro[4.5]dec-8-yl)-3-morpholin-4-yl-2-pyridin-4-yl-propenthione (374.5 mg, 1 mmol) and 2-bromo-1-(4-chloro-phenyl)-ethanone (233 mg, 1 mmol), the title product was prepared in 29% yield as described in Example 1C.
[0135] 1 H NMR
[0136] (CDCl 3 )δ8.62-8.58 (m, 2H), 7.78-7.74 (m, 2H), 7.52-7.45 (m, 5H), 3.98 (s, 1H), 3.24 (t, J=5.7Hz, 4H), 1.83 (t, J=5.8Hz, 4H); MS (m / z): 441 (MH + );
[0137] HRMS: C 23 h 21 ClN 2 o 3 MH of S + The theoretical value is 441.1039; the measured value is 441.1025.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com