Treatment of pulmonary hypertension by inhaled iloprost with a microparticle formulation
A technology of iloprost and medicament, which is applied in the field of treatment of pulmonary arterial hypertension, and can solve problems such as side effects and limited effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0236] If one surfactant is used during the preparation of the matrix, various surfactants can be added to the continuous phase such as emulsifiers. Exemplary emulsifiers or surfactants used can be (0.1-5% by weight), including most physiologically acceptable emulsifiers. Examples include bile salts or bile acids, conjugated amino acids and non-conjugated ones such as bezoar deoxycholic acid, and natural and synthetic forms of cholic acid. In contrast to the method described here, these surfactants will coat the microparticles and facilitate the dispersion of the administration.
[0237] Pore former
[0238] An amount between 0.01% and 90% weight / volume can be included to increase matrix porosity and pore generation during matrix preparation. The pore-forming agent can be added to, for example, solid particles or an aqueous solvent to the polymer solution, emulsified with the polymer solution or co-dissolved in the polymer solution. For example, in spray drying, solvent evapora...
Embodiment 1
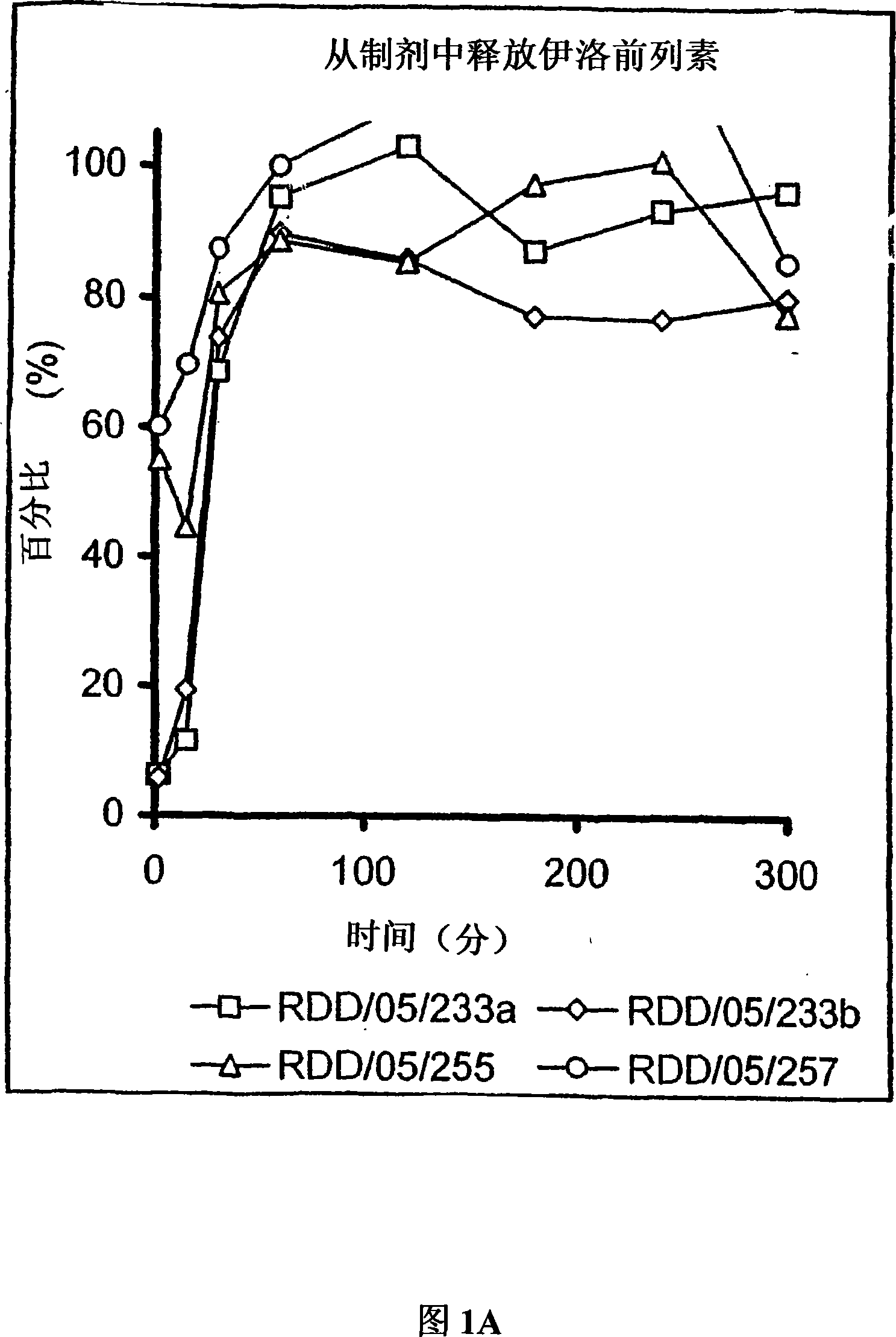
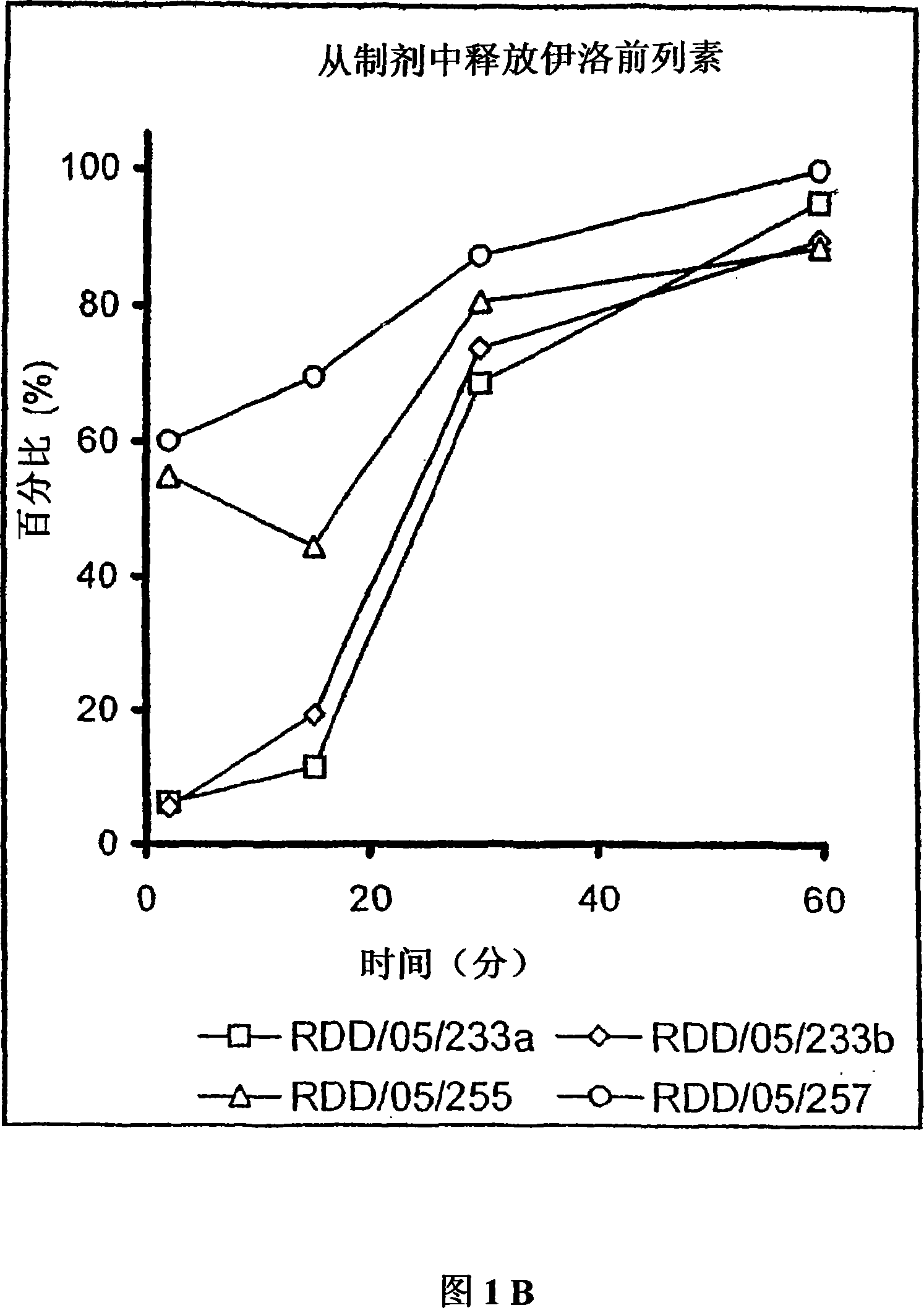
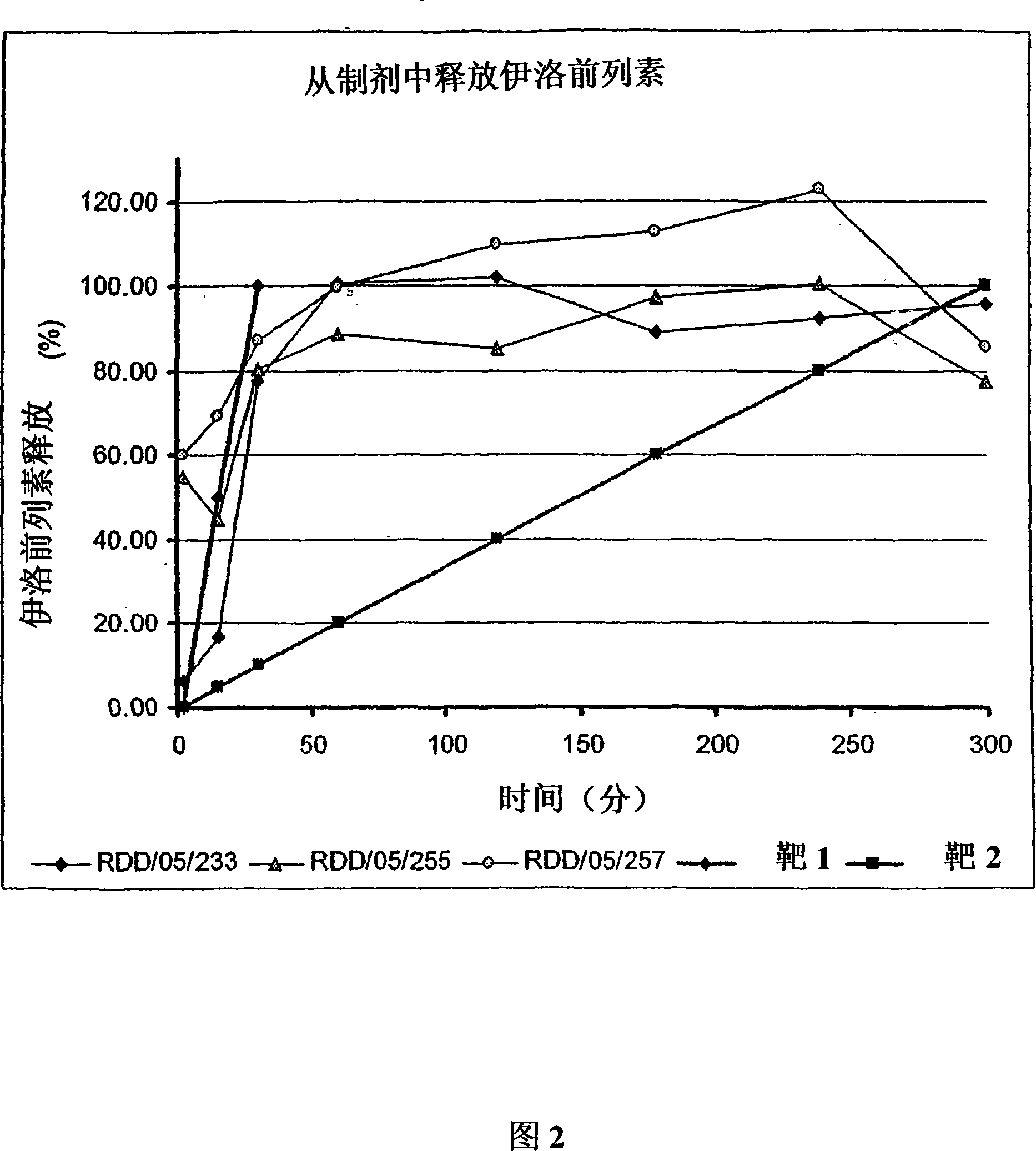
[0598] Microparticle porosity and iloprost or another drug other than iloprost that need to be given release In vitro analysis of effects
[0599] To prepare microspheres containing iloprost and / or another drug that needs to be administered in addition to iloprost, the raw materials used are obtained by the following method: iloprost is obtained from Schering AG or another suitable supplier Obtained; Phospholipids (DPPQ from Avanti Polar Lipids (Alabaster, AL) or another suitable supplier; Polymer (PLGA) from BI Chemicals (Petersburg VA) or another suitable supplier; Ammonium bicarbonate from Spectrum Chemicals (Gardena CA); and dichloromethane from EMScience (Gibbstown NJ) or another suitable supplier.
[0600]Use any of the above-mentioned different combinations of particle components to prepare microparticles containing iloprost and / or another drug other than iloprost that need to be administered and having different porosities, so that the resulting microparticles have diffe...
Embodiment 2
[0603] Containing iloprost for in vivo analysis and / or another drug that needs to be administered in addition to iloprost Preparation of radiolabeled microparticles
[0604] The microparticles containing iloprost and / or another drug to be administered in addition to iloprost were prepared as in the method of the above-mentioned Example 1.
[0605] The dried microspheres are then labeled with technetium or another suitable isotope. In addition, other suitable detectable labels can also be used. The labeled particles are transferred to a stainless steel mixing container and artificially mixed with lactose. The mixed raw materials are then mixed with a turbulent vibration-mixer, and the mixed materials are manually filled into capsules, such as No. 3 Coni-Snap capsules obtained from Capsugel, Greenwood, S.C. or other suitable capsules.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com