Injection for mastitis
An injection and mastitis technology, applied in the field of injections for mastitis, can solve the problems of increased dosage of the main agent, low dissolution, and low absorption of the main agent, and achieves improved absorption, short residual time, and improved dissolution. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] An injection agent 1 (3 g) having a composition in Table 1 was prepared using octanoic acid monoester Sunsoft 707 (manufactured by Sun Chemical Co., Ltd.) having 8 carbon atoms as MCM.
[0036] [Table 1]
[0037] Injection 1
[0038] Main ingredient Cefazolin (CEZ) 150mg (potency)
[0039] MCM 75mg (2.5% by weight)
[0040] Additive Edible Blue No. 1 25mg
[0041] Oily base rapeseed oil balance
[0042] Total 3g
[0043] The above-mentioned injection 1 was filled into a plastic syringe for mastitis ointment commonly used by the applicant company, and an injection for mastitis was trial-produced. The injection preparation 1 prepared as a trial showed physical properties suitable for use as an injection preparation for mastitis.
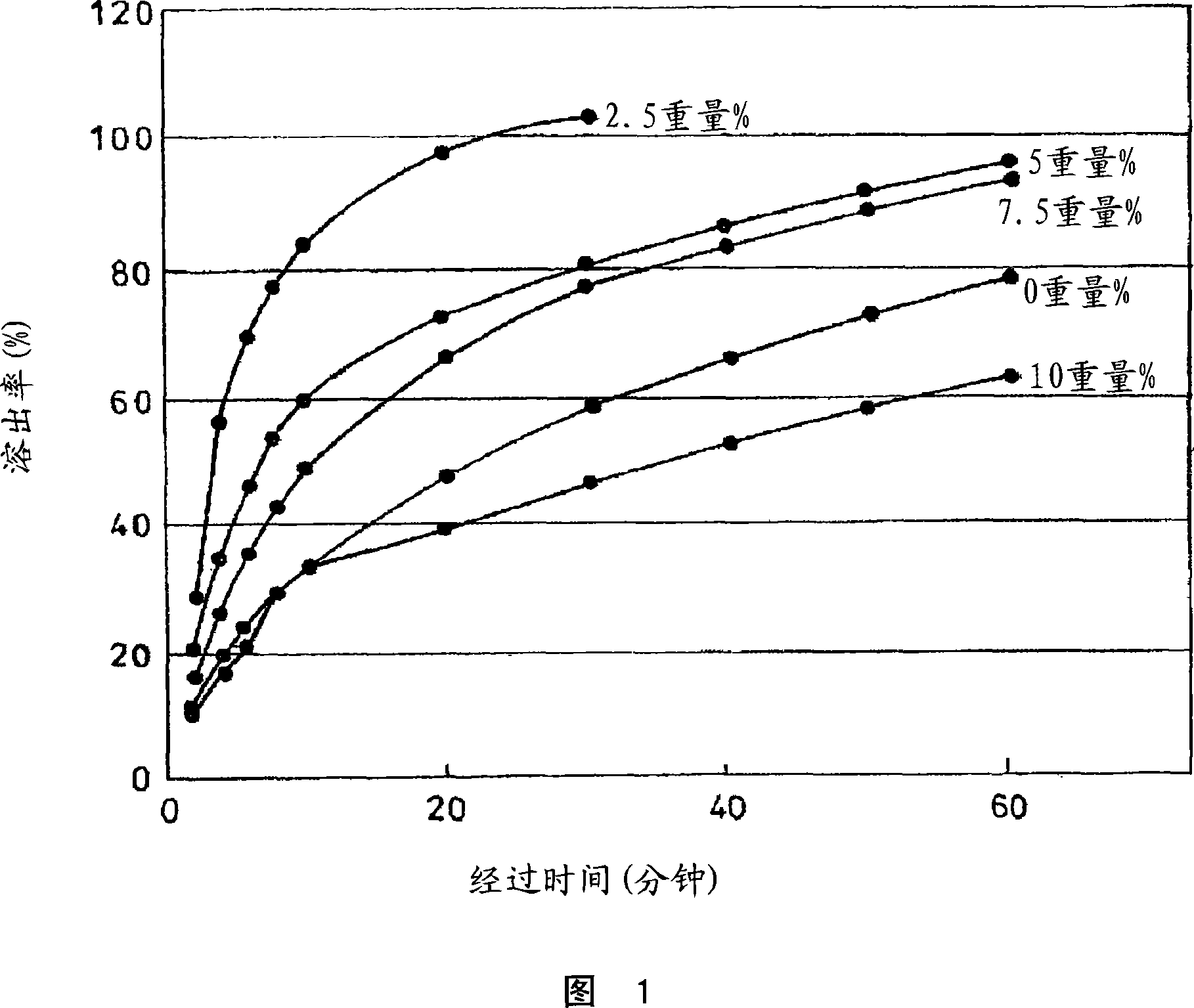
[0044] 1. Dissolution test carried out by changing the ratio of MCM
[0045] For injection 1, according to the dissolution test method No. 2 (paddle method) of the Japanese Pharmacopoeia General Test Method, phosphate buffer (pH 6.5) (4.8...
Embodiment 2
[0104] [Table 4]
[0105] Injection 2
[0106] Main ingredient Cefazolin (CEZ) 75mg (potency)
[0107] MCM 75mg (2.5% by weight)
[0108] Additive Edible Blue No. 1 25mg
[0109] Oily base rapeseed oil balance
[0110] Total 3g
[0111] The above-mentioned injection 2 was filled into a plastic syringe for mastitis ointment commonly used by the applicant company, and an injection for mastitis was trial-produced. The injection preparation 2 prepared as a trial showed physical properties suitable for use as an injection preparation for mastitis.
Embodiment 3
[0113] [table 5]
[0114] Injection 3
[0115] Main ingredient Cefazolin (CEZ) 50mg (potency)
[0116] MCM 75mg (2.5% by weight)
[0117] Additive Edible Blue No. 1 25mg
[0118] Oily base rapeseed oil balance
[0119] Total 3g
[0120] The above injection 3 was filled into a plastic syringe for mastitis ointment commonly used by the applicant company, and the injection for mastitis was trial-produced. The trial-produced injection 3 exhibited physical properties suitable for use as an injection for mastitis.
[0121] 1. Dissolution test carried out by changing the ratio of MCM
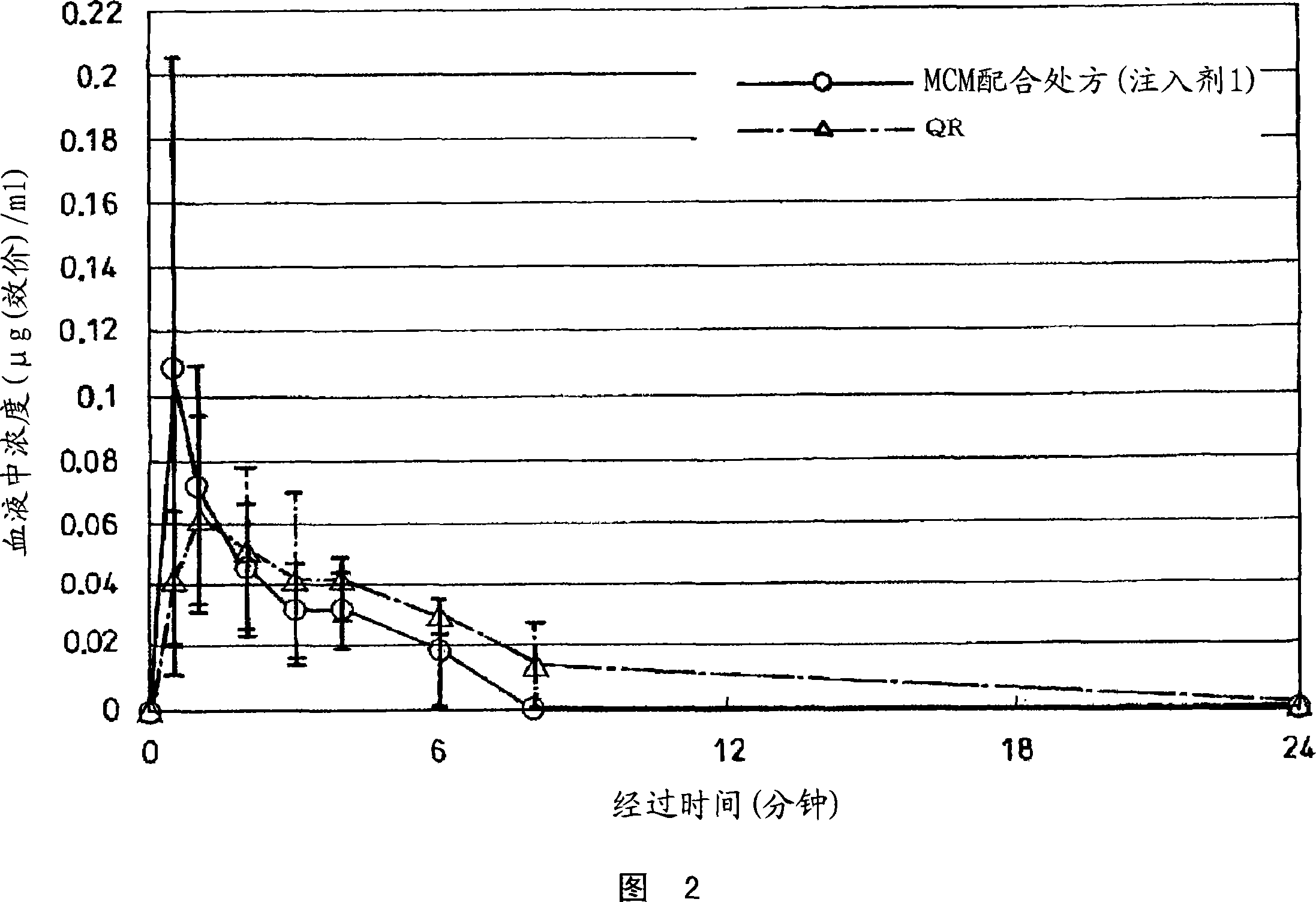
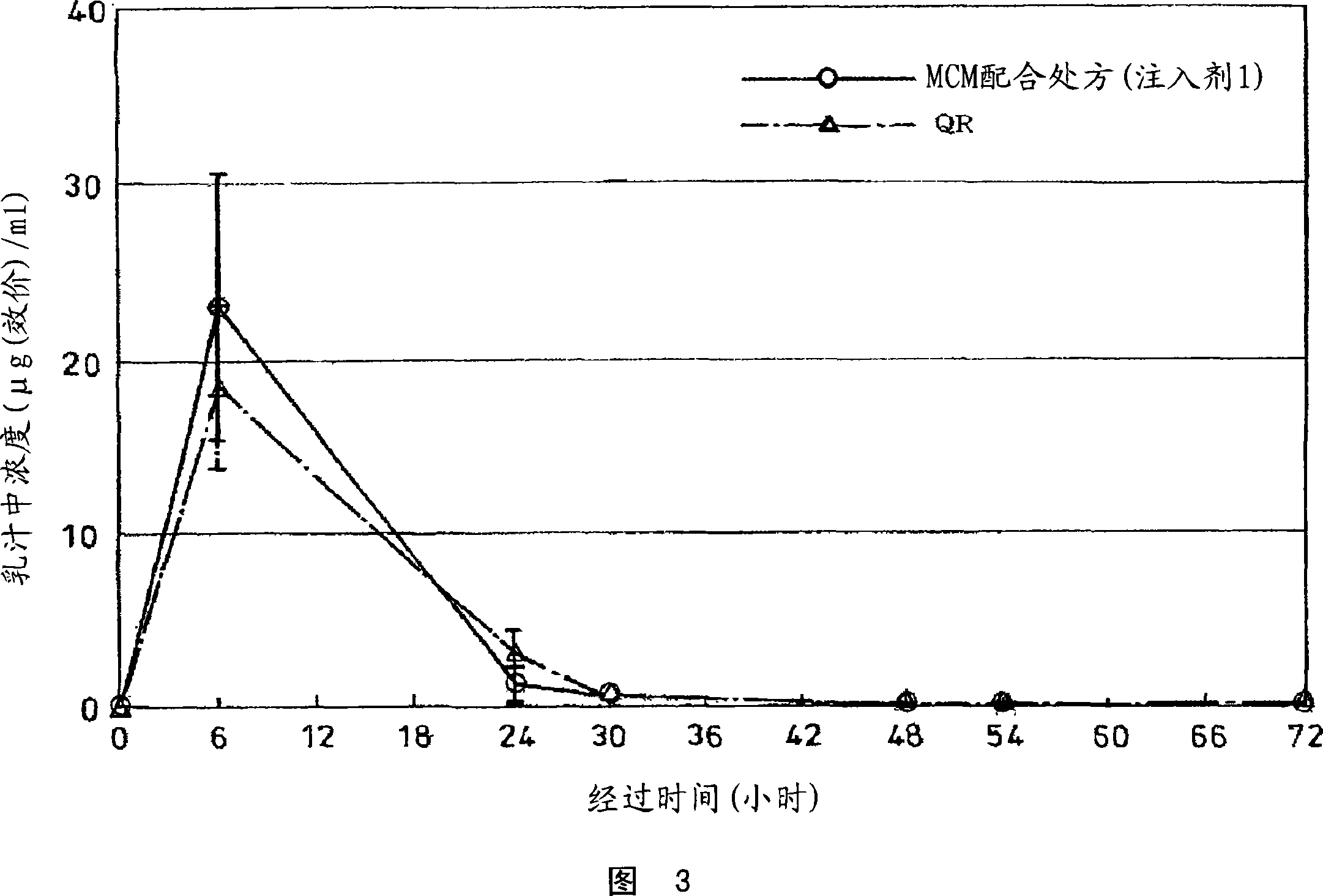
[0122] Figure 4 shows the dissolution rate of the injection 1, injection 2, injection 3 and the control drug Sephamethin QR, and the dissolution rate is shown in Figure 5. As shown in Fig. 5, the injection 3 of the CEZ1 / 3 formulation containing MCM started to dissolve immediately, and dissolved about 100% in 10 minutes. About 100% of CEZ containing MCM was dissolved in 40 minutes with the same ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap