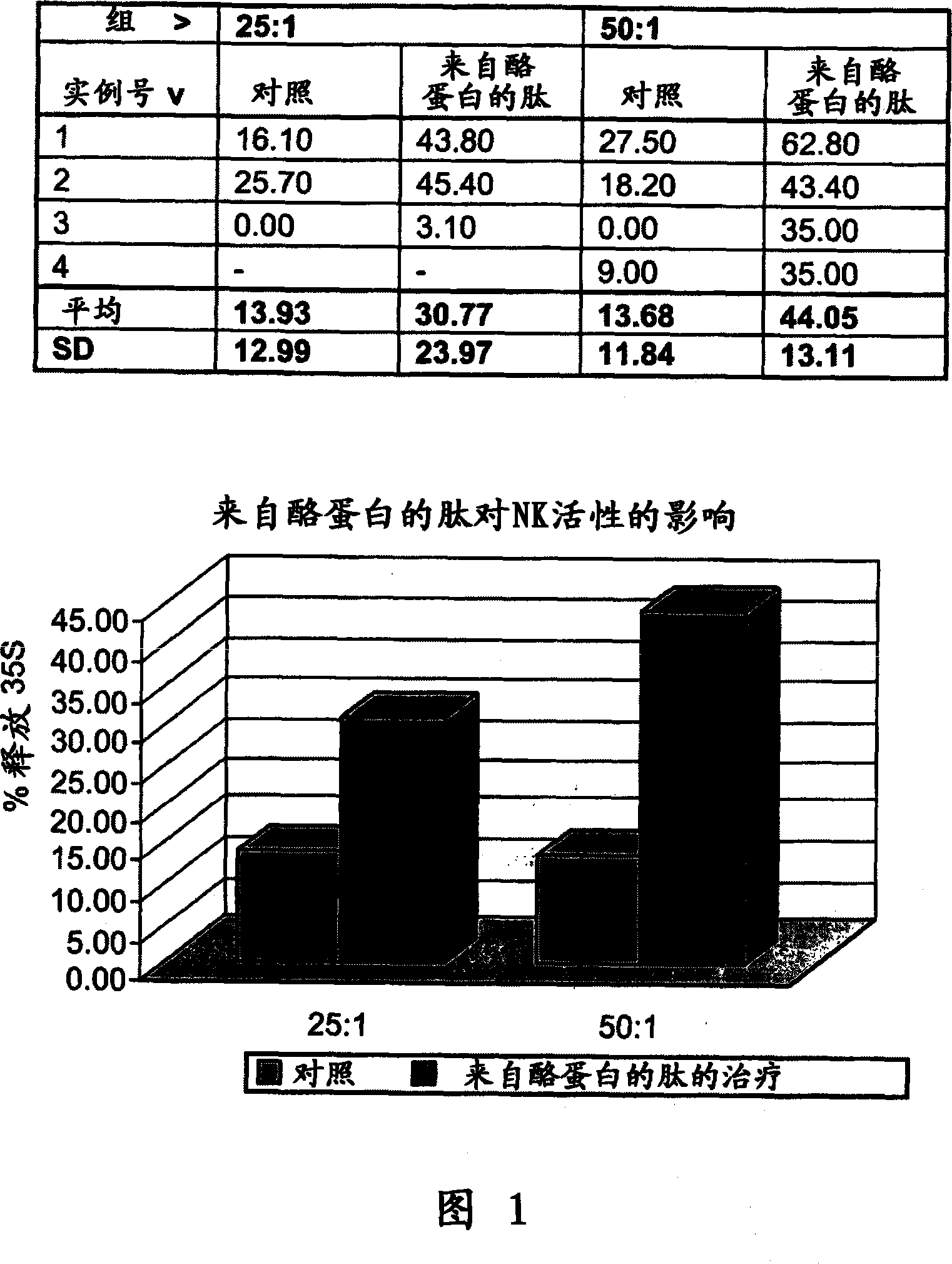
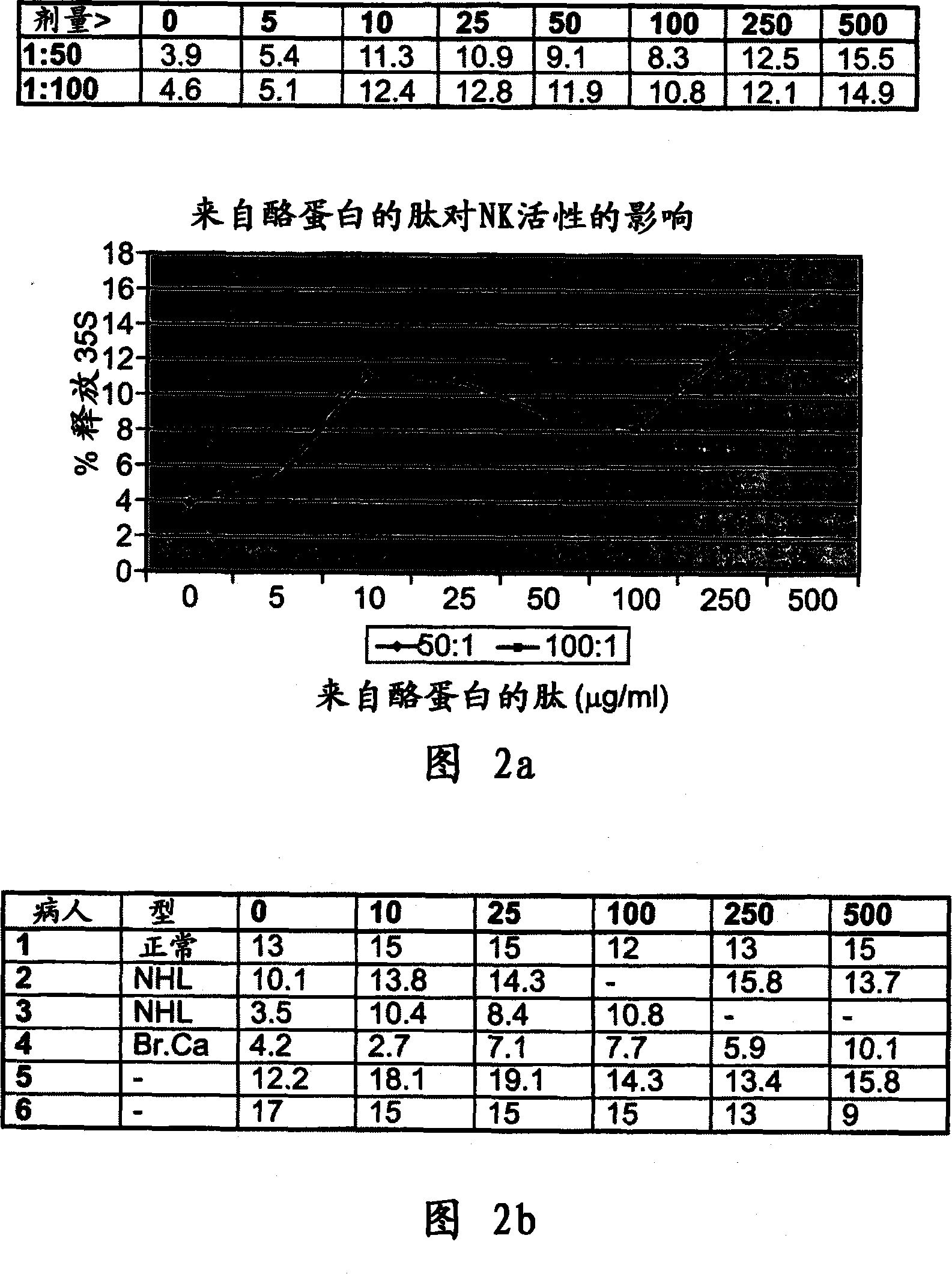
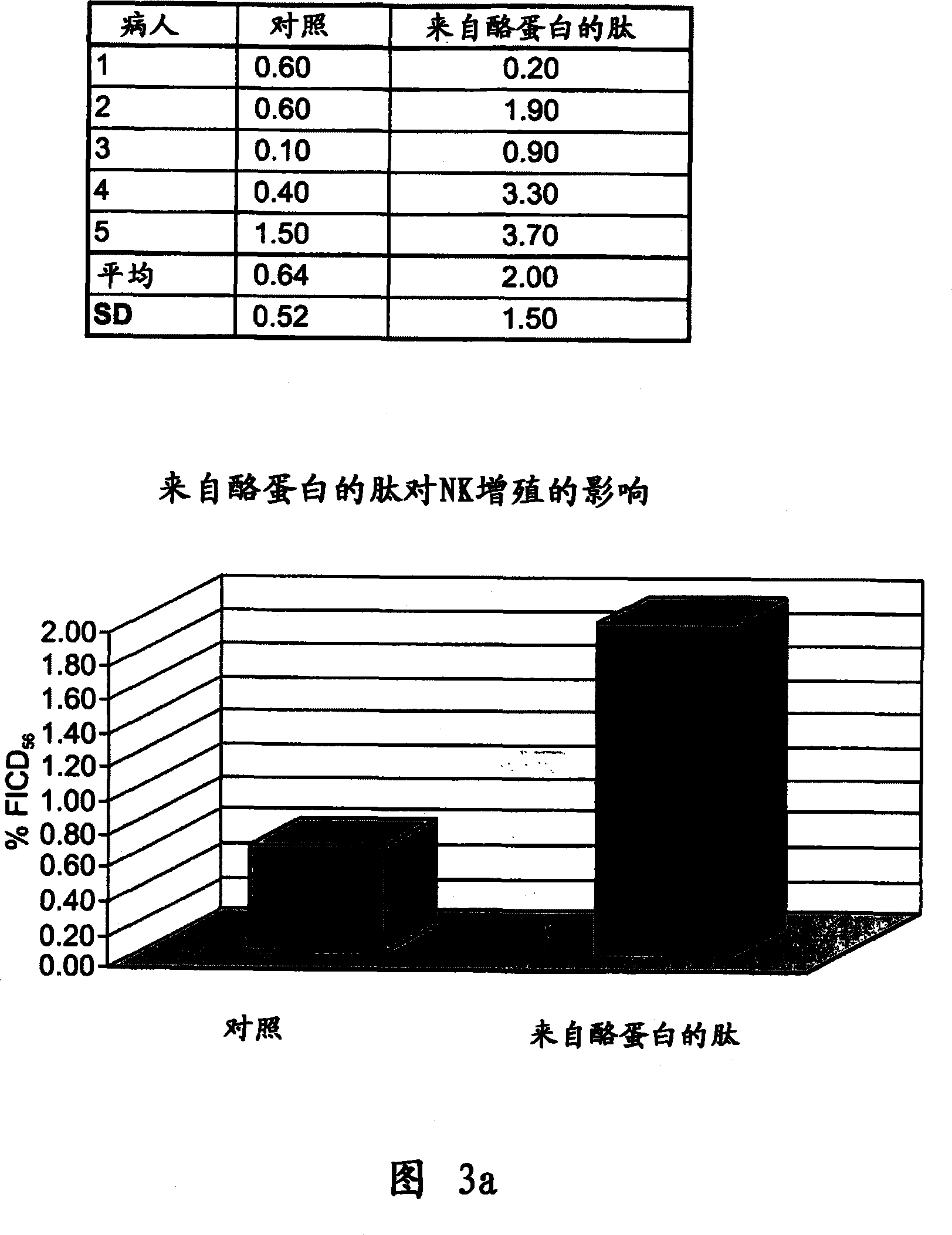
Casein derived peptides and therapeutic uses thereof
A casein, chimeric peptide technology, applied in the fields of peptide/protein composition, animal protein processing, peptide, etc., can solve the problem of not mentioning the application of protein fragments
Inactive Publication Date: 2008-02-13
PEPTERA PHARMA LTD
View PDF10 Cites 21 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0029] Previous studies have documented that potentially bioactive peptides are encoded in the N-terminus of αS1-casein, αS2-casein, β-casein, and κ-casein in the amino acid sequences, but did not mention the application, specific Sequence or defined synthetic peptides, alone or in combination, to enhance hematopoiesis, protect against viral infection, or modulate the development of autoimmune diseases
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment
[0310] Reference is now made to the following examples, which together with the above description explain the invention in a non-limiting form.
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Login to View More
Abstract
Biologically active peptides that are derived from or are similar to sequences of the alphaS1-, alphaS2-, beta- or kapa-casein fractions of milk casein. These peptides are capable of immune modulation and other therapeutic activities, including but not limited to stimulating and enhancing immune response, protecting against viral infection, normalizing serum cholesterol levels, and stimulating hematopoiesis. The casein-derived peptides are non-toxic and can be used to treat and prevent immune pathologies, diabetes, hypercholesterolemia, hematological disorders and viral-related diseases.
Description
Technical field [0001] The present invention relates to biologically active peptides derived from or similar to the sequence of αS1-, αS2-, β- or κ-casein fragments of casein. These peptides can immunomodulate and other therapeutic activities, including but not limited to stimulating and enhancing immune responses, preventing viral infections, normalizing serum cholesterol levels, and stimulating hematopoiesis. Casein-derived peptides are non-toxic and can be used to treat and prevent immunopathology, diabetes, hypercholesterolemia, hematopoietic diseases and virus-related diseases. Background technique [0002] Bioactive molecules from nutrients: [0003] In addition to the nutritional value of many foods, certain fragments and products of the digestive pathway have the ability to affect physiological processes. Some of these "extra-nutritive" ingredients are present in their entire nutrients in their active form, such as immunoglobulins in breast milk and colostrum, phytoestrog...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C08H1/04A23J3/10A61K38/16A61K38/00
CPCA61K38/00C07K14/4732A61P3/00A61P3/06A61P3/08A61P3/10A61P7/00A61P7/04A61P7/06A61P31/00A61P31/04A61P31/12A61P31/18A61P37/00A61P37/04A61P37/06A61P43/00A61K38/17C07K14/435
Inventor Z·斯德尔曼
Owner PEPTERA PHARMA LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com