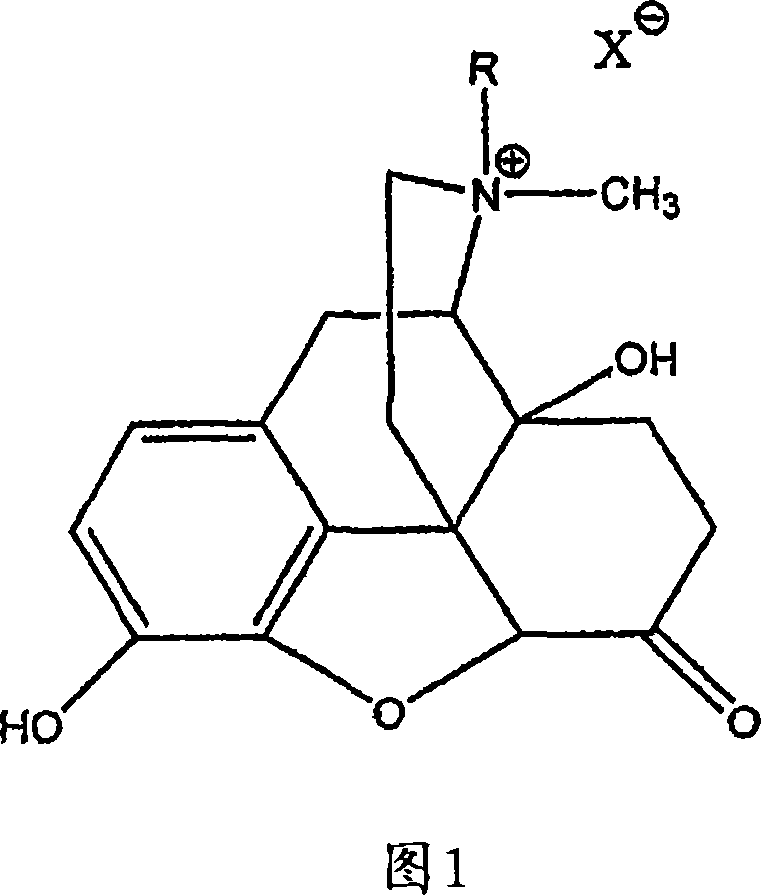
Use of methylnaltrexone and related compounds to treat post-operative gastrointestinal dysfunction
A technology of methylnaltrexone and medroxymorphone, which is applied in the field of compositions for the treatment of postoperative gastrointestinal dysfunction, and can solve problems such as inconsistency and lack of statistical significance in endpoint studies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Example 1: Study Design
[0094]The study was a double-blind, randomized, parallel-group study designed to evaluate the safety and activity of IV (intravenous) MNTX in the treatment of patients undergoing laparotomy for partial colectomy and the duration of their postoperative ileus. Patients were randomly assigned to placebo (saline) or a fixed dose of IV MNTX 0.30 mg / kg patient body weight in 50cc 0.9% normal saline, injected into a large vein with IV piggyback (IVPB) and Infuse for 20 minutes every 6 hours until 24 hours after the patient can tolerate solid food, until the patient is discharged from the hospital, or for a maximum of 7 days. These time points for study drug discontinuation were chosen to allow analysis of the secondary efficacy endpoints of IV MNTX as well as the primary endpoint.
[0095] 65 patients were enrolled in approximately 8 study centers. This suggested sample size has approximately 80% power to test for differences between treatment gro...
Embodiment 2
[0116] Example 2: Treatment of Patient
[0117] Screening (up to 2 weeks prior to surgery) All patients meeting eligibility criteria were explained to the study and given informed consent at their preoperative visit. Obtained for all patients including vital signs, detailed medical history, laboratory tests (Chemistry panel and complete blood count (CBC) with differential and including platelets), 12-lead supine, resting ECG, and all past and Physical exam including current drug check. Females of childbearing potential had a negative urine pregnancy test at least 2 days prior to dosing.
[0118] Day of Surgery Within 24 hours of surgery, patients were randomized to MNTX 0.30 mg / kg or placebo (saline) dose.
[0119] Pre-Induction Care All patients had IV catheters inserted on arrival at the pre-induction area or operating room. After induction, all patients received 7-10 ml / kg intravenous fluid every hour.
[0120] Anesthesia Care Prior to anesthesia, patients received mi...
Embodiment 3
[0136] Example 3. Research steps
[0137] Perform the following steps for each patient, as described below:
[0138] Physical exam / vital signs (screening and end of treatment or premature termination visit)
[0139] ●Laboratory evaluation (screening and end of treatment or premature termination visit)
[0140] ●Electrocardiogram (ECG)
[0141] ●Vital signs before and after administration
[0142] ● Verbal Numeral Scale / Vomiting Assessment
[0143] ●Presence or absence of nasogastric tube (NGT) or orogastric tube (OGT)
[0144] ●Liquid to try
[0145] Regardless of bowel sounds, at each visit to the patient at 7 am, 12 pm, 5 pm, and 10 pm (±30 minutes) beginning at the first scheduled time point in the postoperative morning, a qualified study designator passed the patient Give the patient 30 cc of water (measured with an oral syringe) through the mouth until the patient can tolerate the 30 cc of fluid. Intolerance to clear fluids was prescribed as nausea and / or vomitin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com