Fumagillol derivatives or method for preparation of fumagillol derivatives, and pharmaceutical compositions comprising the same
A technology of fumagillol and derivatives, applied in drug combination, antitumor drugs, organic chemistry and other directions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
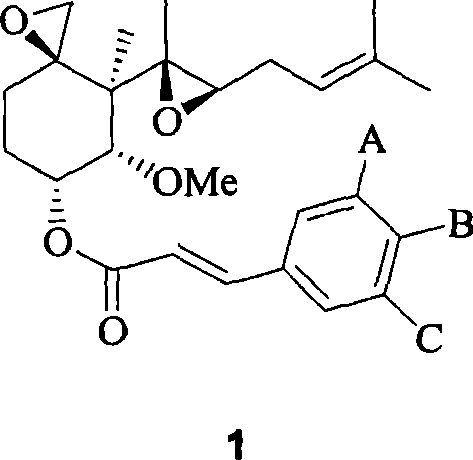
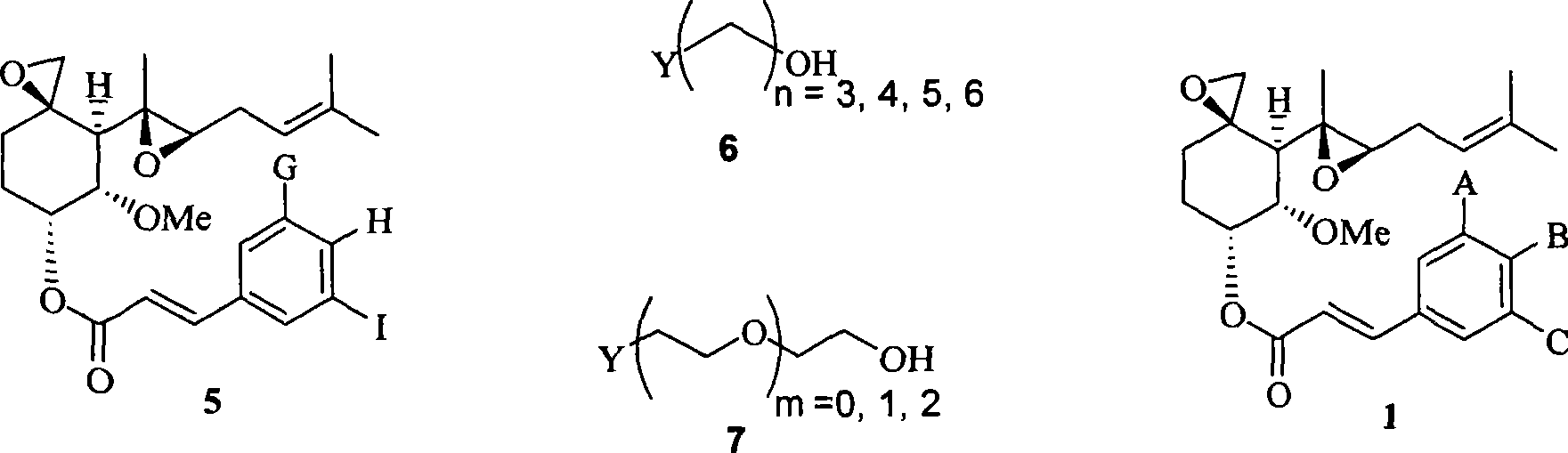
[0091] Example 1: Preparation of O-(4-(2-hydroxyethoxy)cinnamoyl) fumagillol
[0092] Step 1: Preparation of O-(4-acetoxycinnamoyl) fumagillol
[0093] 4-acetoxycinnamic acid (1.825 g, 8.85 mmol) was stirred in toluene (20 ml), thionyl chloride (1.29 ml, 1.77 mmol) was added dropwise thereto, and the resulting mixture was stirred under reflux for 4 hours. The solvent was evaporated under reduced pressure, and the residue was dissolved in dimethylformamide (20 ml). Sodium hydride (850 mg, 21.25 mmol) and the compound of Chemical Formula 2 (1.0 g, 3.54 mmol) were added dropwise thereto, and the resulting mixture was then stirred at normal temperature for 4 hours. This solution was added to a saturated aqueous ammonium acetate solution (200 ml), and then extracted with ethyl acetate (250 ml). The organic layer was washed 3 times with a saturated saline solution (200 ml). The organic layer was dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure. T...
Embodiment 2
[0101] Example 2: Preparation of O-(3,5-dimethoxy-4-(2-hydroxyethoxy)cinnamoyl)fumagillol
[0102] Step 1: Preparation of O-(4-acetoxy-3,5-dimethoxycinnamoyl) fumagillol
[0103] Repeat the same method described in step 1 of Example 1, but using the compound of formula 2 (1.0g), 4-acetoxy-3,5-dimethoxycinnamic acid (2.36g), thionyl chloride (1.29 ml), toluene (20ml), sodium hydride (850mg) and dimethylformamide (20ml) to give 1.36g (72%) of the title compound as a white solid.
[0104] 1 H-NMR (400MHz, CDCl 3 )δ: 7.59 (d, 1H, J = 16Hz), 6.77 (s, 2H), 6.44 (d, 1H, J = 16Hz), 5.71 (m, 1H), 5.21 (m, 1H), 3.86 (s, 3H), 3.71 (dd, 1H, J = 11, 2.7 Hz), 3.45 (s, 3H), 3.0 (d, 1H, J = 4.0 Hz), 2.62 (t, 1H, J = 6.4 Hz), 2.57 ( d, 1H, J = 4.0 Hz), 2.36 (m, 1H), 2.34 (s, 3H), 2.20-2.04 (m, 4H), 1.89 (m, 1H), 1.74 (s, 3H), 1.65 (s , 3H), 1.23(s, 3H), 1.10(m, 1H).
[0105] Step 2: Preparation of O-(3,5-dimethoxy-4-hydroxycinnamoyl) fumagillol
[0106] Repeat the same method described in step 2 o...
Embodiment 3
[0111] Example 3: Preparation of O-(4-(2-hydroxyethoxy)-3-methoxycinnamoyl) fumagillol
[0112] Step 1: Preparation of O-(4-acetoxy-3-methoxycinnamoyl) fumagillol
[0113] Repeat the same method described in step 1 of Example 1, but using the compound of formula 2 (1.0g), 4-acetoxy-3-methoxycinnamic acid (2.09g), thionyl chloride (1.29ml), Toluene (20ml), triethylamine (2.7ml) and dichloromethane (20ml) gave 1.0g (56%) of the title compound as a bright yellow syrup.
[0114] 1 H-NMR (400MHz, CDCl 3 )δ: 7.62(d, 1H, J=16Hz), 7.13-7.03(m, 3H), 6.44(d, 1H, J=16Hz), 5.73(m, 1H), 5.43(m, 1H), 5.21( m, 1H), 3.88 (s, 3H), 3.71 (dd, 1H, J = 11.2, 2.8 Hz), 3.45 (s, 3H), 3.00 (d, 1H, J = 4 Hz), 2.62 (t, 1H, J=6.3Hz), 2.57(d, 1H, J=4Hz), 2.35(m, 1H), 2.32(s, 3H), 2.20-2.04(m, 4H), 1.89(m, 1H), 1.74(s , 3H), 1.65(s, 3H), 1.23(s, 3H), 1.11(m, 1H).
[0115] Step 2: Preparation of O-(4-hydroxy-3-methoxycinnamoyl) fumagillol
[0116] Repeat the same method described in step 2 of Example 1, but usi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com