Method of preparing 3-cyano-quinolines and intermediates made thereby
A cyanoquinoline and aryl technology, applied in the field of preparation of substituted 3-cyanoquinolines, can solve problems such as no quinoline catalysis or elaboration of mild methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
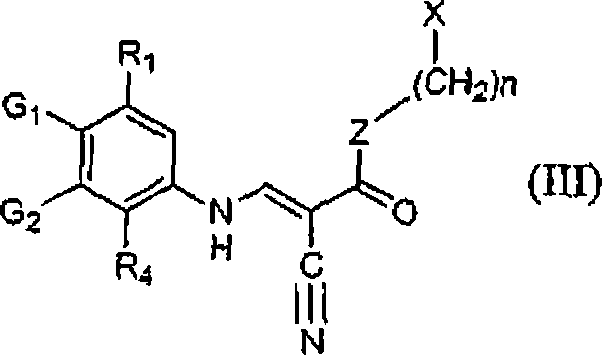
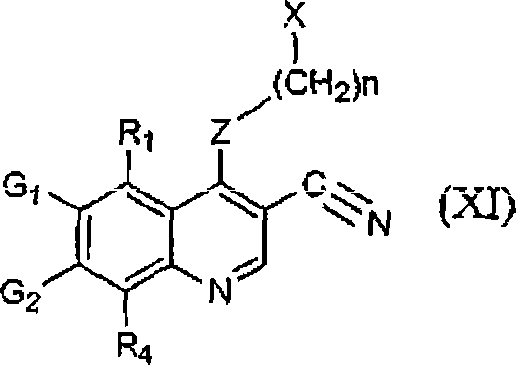
[0087] As mentioned above, the method for preparing 3-cyanoquinolines utilizes two separate routes. Both routes involve the reaction of arylamines, orthoformates, and reactive methylene groups and both lead to the production of N-aryl-2-propene derivatives. Since the method of the present invention does not involve heating to high temperature or use of microwave radiation, it avoids many obstacles of previous synthetic routes. Therefore, the present method can be easily adapted to large-scale preparation of 3-cyanoquinolines. Furthermore, the chlorination step used in previous synthetic methods is avoided. This is an improvement since chlorination of quinolines is known to form various tars and decomposition products, which lead to lower yields of the desired product and difficult to remove impurities. Therefore, syntheses involving quinoline chlorination are extremely unsuitable for large-scale synthesis of 3-cyanoquinolines. The method of the present invention is advantag...
example
[0154] The following examples are provided to illustrate the invention, not to limit the invention.
example 1
[0156] (E / Z)-3-(4-bromo-3-ethoxyanilino)-N-[3-chloro-4-(2-pyridyl-methoxy)phenyl]-2-cyano- Preparation of 2-acrylamide.
[0157]
[0158] A 3 neck 50ml flask was charged with cyanoacetamide (0.50g, 1.7mmol) in triethylorthoformate (2.45g, 2.75ml, d = 0.89g / ml). The mixture was heated to 50-60°C and acetic anhydride (0.42g, 0.39ml, 4.1mmol, 2.5eq, d=1.08g / ml) was added. The flask was heated to 100-105°C for a minimum of 4 hours and then cooled to 70-75°C. A solution of 3-ethoxy-4-bromoaniline hydrochloride (0.42 g, 1.67 mmol) in isopropanol (5 ml) was added. The mixture was stirred for 3 hours and cooled to room temperature. Water was added and the mixture was extracted with ethyl acetate. The organic layer was washed with brine, dried over sodium sulfate and stripped to dryness. The residue was dissolved in acetonitrile (10ml) and water (10ml) was added dropwise to precipitate the product. The product was filtered on a Buchner funnel to obtain 0.11 g of the title comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com