Solid compositions for treating middle-of-the-night insomnia and method therefor
A composition, subject's technology, applied in the field of TN insomnia, capable of solving the problems of excess, administration about 7 to 9 hours before bed, slow sleep, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0139] Those skilled in the art will recognize that the preparation of a gum base need not begin with its individual components. For example, gum bases can be purchased that contain the desired ingredients therein, and can be modified to include additional agents. There are several manufacturers of gum bases suitable for use in such chewing gum compositions. Examples of such gum bases include, but are not limited to, Pharmagum TM M, S, or C (SPIPharma Grollp; New Castle, DE). Usually, Pharmagum TM Contains gum base, sweeteners, plasticizers, and sugar mixtures.
[0140]In some instances, the chewing gum composition includes a therapeutic agent centerfill. Center-fills are particularly suitable when immediate release of the therapeutic agent is preferred. Additionally, encapsulating the therapeutic agent within the center-fill can help mask any undesired taste the therapeutic agent may have. In these cases, the gum base at least partially surrounds the center-fill. The ...
Embodiment 1
[0188] Example 1. Low Dose Zolpidem Lozenge Composition
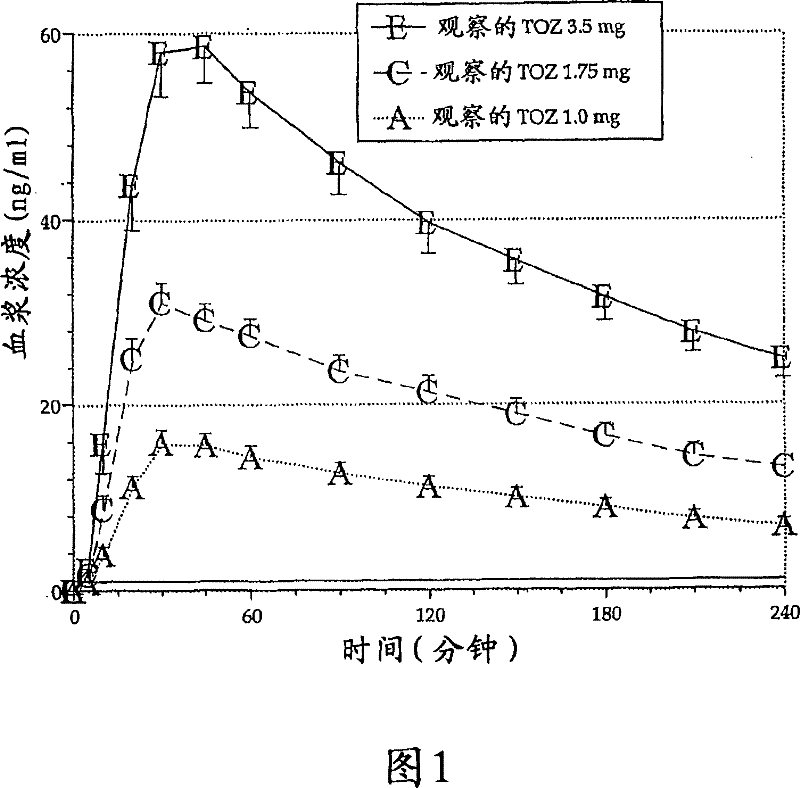
[0189] Sublingual delivery lozenges containing 0 mg, 1.0 mg, 1.75 mg or 3.5 mg zolpidem prepared according to the formulation listed in Table 3 were administered to subjects suffering from midnight insomnia.
[0190] Table 3. Low-Dose Zolpidem Lozenge Formulations
[0191]
[0192]
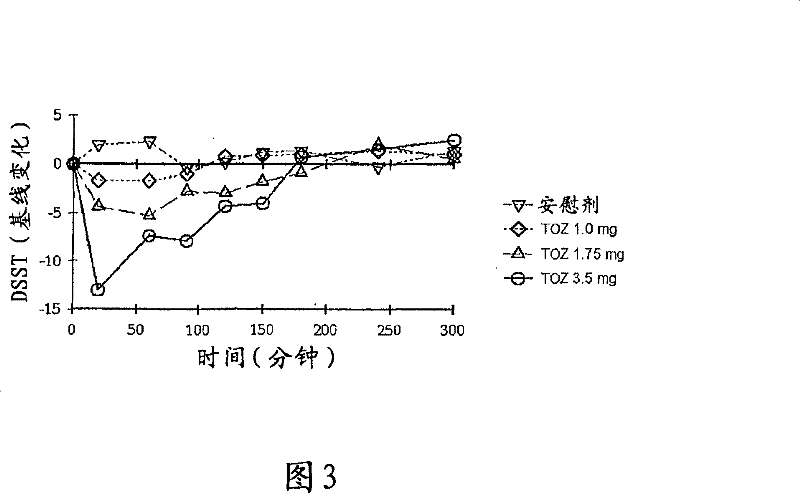
[0193] These individuals self-administered one of the above formulations when their sleep was interrupted and they had at least 2 hours of sleep time remaining. Upon awakening, the individuals provided a subjective self-assessment of any residual sedation and were given the following psychomotor and memory tests to assess any residual sedation: Digit Symbol Substitution Test (DSST), Choice Reaction Time Test (CRT) , Symbol Reproduction Test (SCT) and Buschke memory recall test.
[0194] Individuals who received the placebo were generally unable to fall back to sleep and therefore did not feel refreshed in the morning. Individuals r...
Embodiment 2
[0195] Example 2. Study on pharmacokinetics and pharmacodynamics of low-dose zolpidem lozenge composition
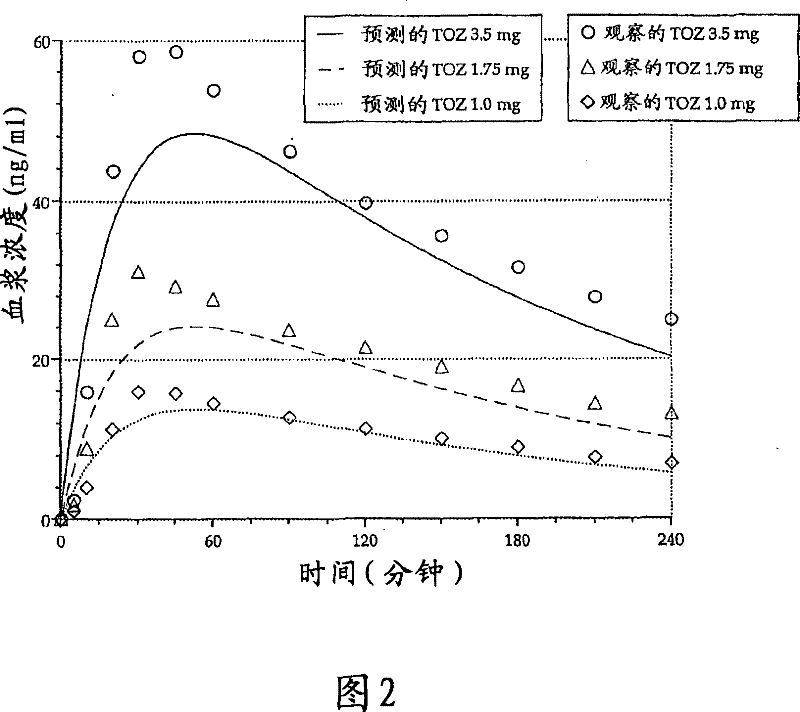
[0196] This example provides an evaluation of the daytime dose-dependent pharmacokinetic and pharmacodynamic effects of the 1.0 mg, 1.75 mg and 3.5 mg zolpidem lozenges described in Table 3 above.
[0197] overview
[0198] Currently, no drug is available, when needed, for patients with middle of the night (MOTN) awakenings and difficulty falling back to sleep. Therapeutic agents suitable for such insomnia enable the patient to return to sleep quickly and have no residual effect upon waking in the morning. In particular, this study demonstrates that the low-dose zolpidem lozenge of the present invention enhances the rapid systemic absorption of zolpidem without affecting other pharmacokinetic parameters.
[0199] This study is a double-blind, placebo-controlled four-group cross-over study. Two consecutive mornings, healthy adults (n=24; average age=37.6 years old) who ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com