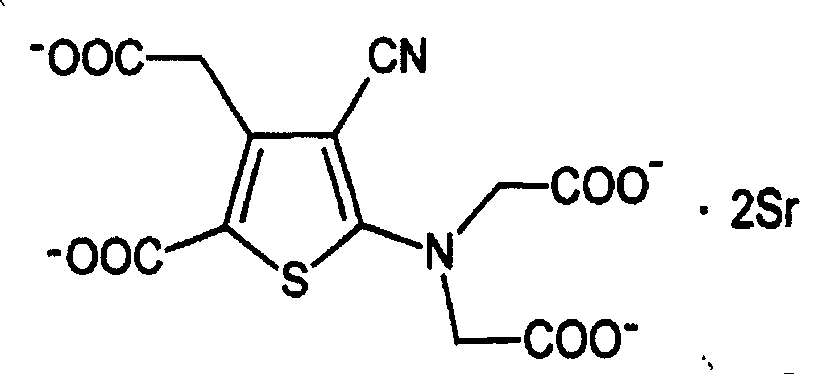
Strontium ranelate dry suspension
A technology of strontium ranelate and dry suspension, applied in bone diseases, powder delivery, drug combination, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Prescription composition:
[0021]
[0022] Preparation technology: take by weighing the raw and auxiliary materials of the recipe quantity, pass through a 100-mesh sieve, and make starch into 15% starch slurry for subsequent use; first mix strontium ranelate and mannitol to obtain mixture I; then mix mixture I with xanthan gum, Sodium carboxymethyl starch is mixed uniformly according to the equal amount incremental method, and an appropriate amount of 15% starch slurry is added to make granules, dried at 50°C, granulated, and graded to obtain Granule I, which is mixed with tartrazine, aspartame, and orange essence. The mixture II is uniformly obtained, and the mixture II is divided into a dry suspension to obtain a dry suspension.
[0023] Dry Suspension Check
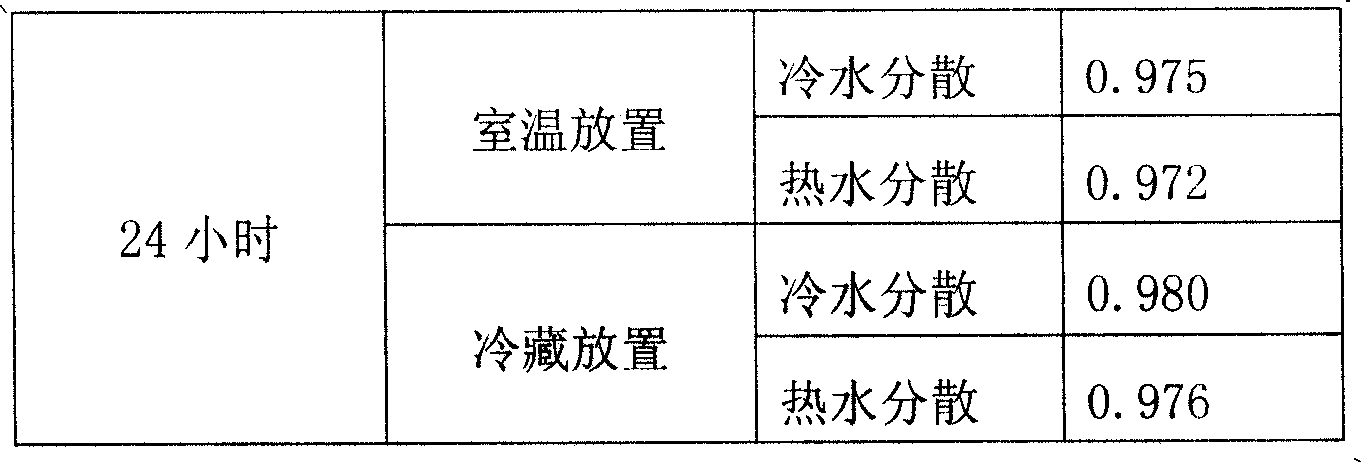
[0024] 1. Check the sedimentation volume ratio: according to the appendix IO method of "Chinese Pharmacopoeia 2005 Edition".
[0025] After dispersing the two bags of particles in 200 ml of cold water and ...
Embodiment 2
[0038]
[0039]
[0040] Preparation technology: take by weighing the raw and auxiliary materials of the recipe quantity, pass through a 100-mesh sieve, and make maltodextrin into 20% maltodextrin pulp for subsequent use; first mix strontium ranelate and mannitol evenly to obtain mixture I; then mix mixture I with Sodium alginate was mixed uniformly by the equal amount incremental method, and an appropriate amount of 20% maltodextrin pulp was added to granulate, dried at 50°C, granulated, and classified to obtain Granule I, which was mixed with fruit green, sucralose and green apple essence. The mixture II is uniformly obtained, and the mixture II is divided into a dry suspension to obtain a dry suspension.
[0041] Dry Suspension Check
[0042] 1. Check the sedimentation volume ratio: according to the appendix IO method of "Chinese Pharmacopoeia 2005 Edition".
[0043] After dispersing the two bags of particles in 200 ml of cold water and 200 ml of hot water, respectiv...
Embodiment 3
[0055] Prescription composition:
[0056]
[0057] Preparation technology: take by weighing the raw and auxiliary materials of the recipe quantity, pass through a 100-mesh sieve, and make starch into 15% starch slurry for subsequent use; first mix strontium ranelate and xylitol homogeneously to obtain mixture I; then mix mixture I with xanthan gum , Sodium carboxymethyl starch is mixed uniformly according to the equal amount incremental method, and an appropriate amount of 15% starch slurry is added to granulate, dried at 50 ° C, granulated, and graded to obtain granules I, and granules I are mixed with tartrazine, aspartame, and orange essence. Mix well to obtain mixture II, and divide the mixture II to obtain a dry suspension.
[0058] Dry Suspension Check
[0059] 1. Check the sedimentation volume ratio: according to the appendix IO method of "Chinese Pharmacopoeia 2005 Edition".
[0060] After dispersing the two bags of particles in 200 ml of cold water and 200 ml of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com