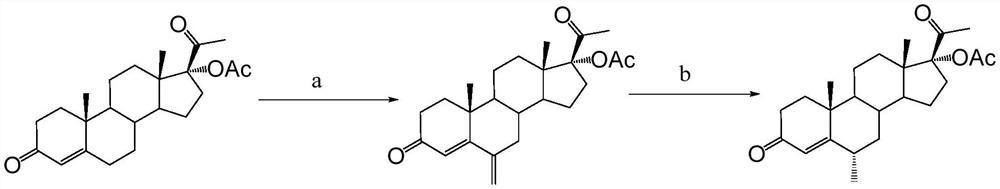
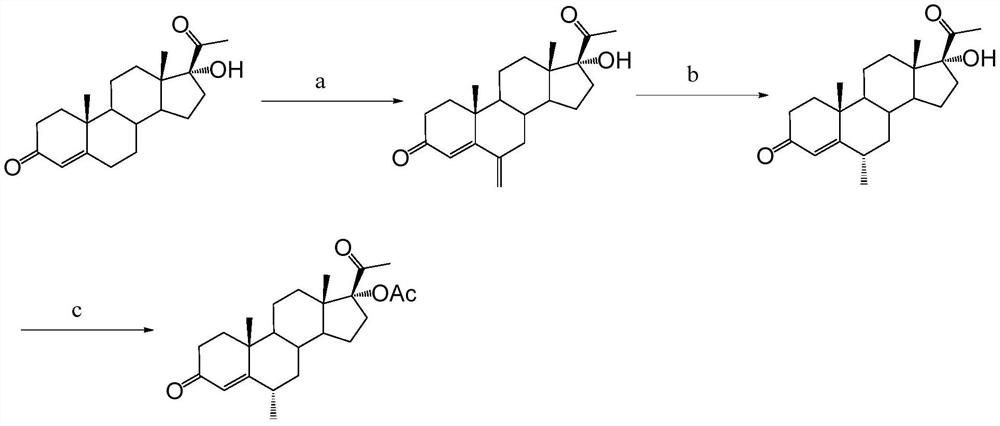
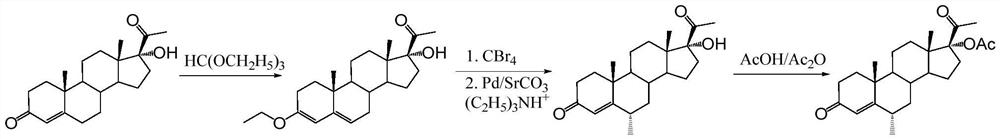Preparation method of medroxyprogesterone acetate for perimenopausal syndrome
A technology of medroxyprogesterone acetate and ketone acetate, applied in the field of medicine and chemical industry, can solve the problems of long time, many reaction steps, low yield and the like, and achieves the effects of low cost, less raw material usage and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] In a reactor equipped with a reflux condenser, a dropping funnel, and a magnetic stirring device, add sodium acetate (3.0 g), aluminum trichloride (266 mg, 2 mmol), 1,3-dioxane (12 ml, about 12.8 g), then added anhydrous chloroform (200ml) and stirred at reflux for 0.5h. Subsequently, 17α-hydroxyprogesterone acetate (3.73g, 10mmol) was added and stirred to dissolve to form a reaction solution, then phosphorus oxychloride (2.8ml, 30mmol) was slowly added dropwise to the reaction solution through a dropping funnel, 1.5 h is added. After the addition, continue to stir the reaction under reflux for 3h. The reaction solution was naturally cooled to room temperature, then a saturated aqueous sodium carbonate solution was added dropwise under vigorous stirring to quench the reaction, the pH of the aqueous layer was monitored, and the addition of a saturated aqueous sodium carbonate solution was stopped when it reached alkalinity (pH was 7-8); Remove the insoluble matter by f...
Embodiment 2
[0054] In a reactor equipped with a reflux condenser, dropping funnel, and magnetic stirring device, add sodium acetate (3.0 g), aluminum trichloride (200 mg, 1.5 mmol), 1,3-dioxane (12 ml, about 13g), then added anhydrous chloroform (200ml) and stirred at reflux for 0.5h. Subsequently, 17α-hydroxyprogesterone acetate (3.73g, 10mmol) was added and stirred to dissolve to form a reaction solution, then phosphorus oxychloride (2.8ml, 30mmol) was slowly added dropwise to the reaction solution through a dropping funnel, 1.5 h is added. After the addition was complete, the stirring reaction was continued under reflux for 3.5h. The reaction solution was naturally cooled to room temperature, then a saturated aqueous sodium carbonate solution was added dropwise under vigorous stirring to quench the reaction, the pH of the aqueous layer was monitored, and the addition of a saturated aqueous sodium carbonate solution was stopped when it reached alkalinity (pH was 7-8); Remove the insol...
Embodiment 3
[0056] In a reactor equipped with a reflux condenser, dropping funnel, and magnetic stirring device, add sodium acetate (3.0 g), aluminum trichloride (200 mg, 1.5 mmol), 1,3-dioxane (15 ml, about 16g), then added anhydrous chloroform (200ml) and stirred at reflux for 0.5h. Subsequently, 17α-hydroxyprogesterone acetate (3.73g, 10mmol) was added and stirred to dissolve to form a reaction solution, then phosphorus oxychloride (2.3ml, 25mmol) was slowly added dropwise to the reaction solution through the dropping funnel, 1.5 h is added. After the addition was complete, the stirring reaction was continued under reflux for 3.5h. The reaction solution was naturally cooled to room temperature, then a saturated aqueous sodium carbonate solution was added dropwise under vigorous stirring to quench the reaction, the pH of the aqueous layer was monitored, and the addition of a saturated aqueous sodium carbonate solution was stopped when it reached alkalinity (pH was 7-8); Remove the ins...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com