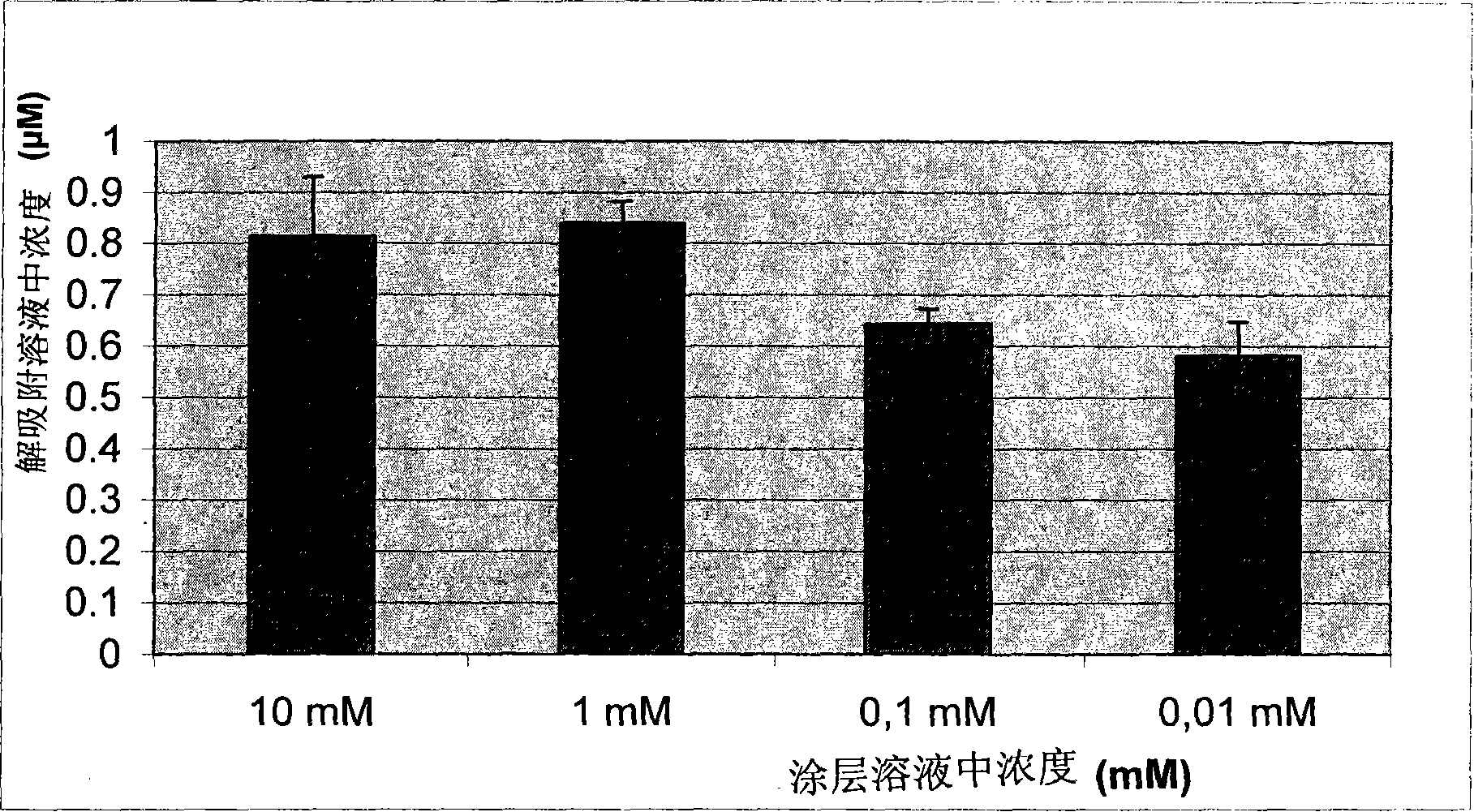
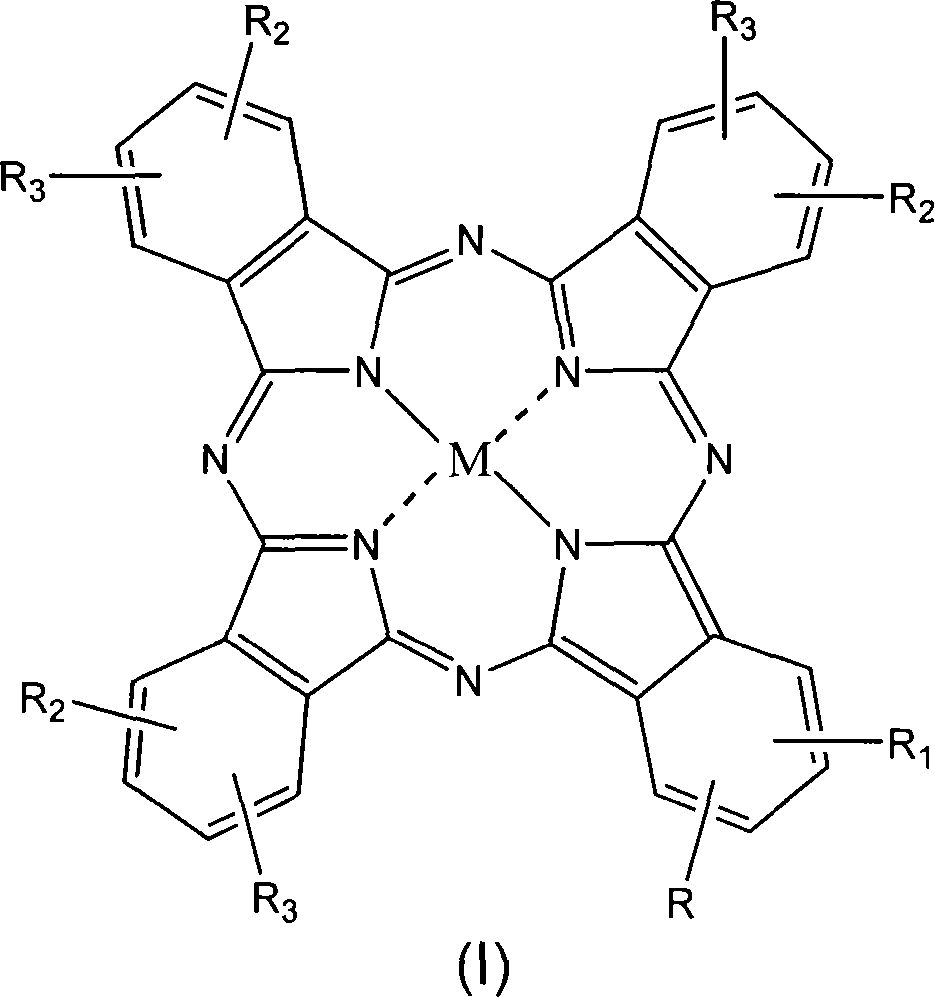
Self-sterilizing products
A product and selected technology, applied in the field of phthalocyanine derivatives, can solve the problem that sterilization is not permanent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0076] Preparation of diiodides of phthalocyanine derivatives of structural formula (I), wherein M is Zn, R 1 = R 2 = R 3 =H, and R=1,3-bis-(trimethylamine)-2-propoxy in position 2 [compound 1]
[0077] 0.272 g of 4-[1,3-bis-(dimethylammonia)-2-propoxyl]-1,2-phthalonitrile (1 mmol) and 0.384 g of 1,2-phthalonitrile (3 mmol) was dissolved in a small amount of methanol; to the resulting solution was added to Zn(AcO) 2 (0.176g; 0.96mmol) and DBU (0.66ml; 0.42mmol). The mixture was heated to 150°C for 3 hours and 30 minutes under an inert atmosphere. The blue mixture was dissolved in DMF and reprecipitated several times with basic water, then purified by flash chromatography on silica gel with Et 2 O / DMF (4:1), EtOAc / DMF (4:1), EtOAc / DMF (1:1), EtOAc / DMF (1:2), and DMF eluted.
[0078] The resulting product is a compound of structural formula (I), wherein M is Zn, R 1 = R 2 = R 3 = H and R = 1,3-bis-(dimethylamino)-2-propoxy in position 2 [compound 1bis]; 10 mg of this pr...
example 2
[0083] Preparation of the phthalocyanine derivative octaiodide of structural formula (I), wherein M is Zn, R 1 = R 2 =H, and R=R 3 = 1,3-bis-(dimethyl-ethyl acetate-amine)-2-propoxy in position 2, 9(10), 16(17), 23(24) [compound 2]
[0084] The title compound was prepared by the following procedure as described in Example 1 starting from 4-[1,3-bis-(dimethylamino)-2-propoxy]-1,2-phthalonitrile to obtain the formula The compound of (I), wherein M is Zn, R 1 = R 2 =H, and R=R 3 = 1,3-bis-(dimethylamino)-2-propoxy in position 2, 9(10), 16(17), 23(24) [compound 2bis].
[0085] 0.5ml of ICH 2 COOEt was added to a solution obtained by dissolving 5 mg of the amino derivative in 1 ml of N-methylpyrrolidone, and the mixture was stirred for 3 days. Product from Et 2 O was precipitated, and the solid phase was washed several times with ether to remove residual solvents and impurities.
[0086] The product is finally absorbed by DMF, by Et 2 O is precipitated, and by Et 2 O and...
example 3
[0090] Preparation of structural formula (I) phthalocyanine derivative diiodide, wherein M is Zn, R 1 = R 2 = R 3 = H, and R = 1,3-bis-(dimethyl-ethyl acetate-ammonium)-2-propoxy in position 2 [Compound 3]
[0091] With the same method described in Example 2, the title compound was prepared, and the NMR analysis results of the gained compound were as follows:
[0092] 1 H-NMR (300MHz, DMSO-d 6 )δ (ppm) 9.5-9.3 (m, 6H), 9.1 (s, 2H), 8.1-8.3 (m, 7H), 6.2 (m, 1H), 4.75 (m, 4H), 4.5 (b.d.2H, J =12Hz), 4.3(b.d.2H, J=12Hz), 4.05(q, 4H, J=10Hz), 3.5(s, 12H), 1.0(t, 6H, J=10Hz). 13 C-NMR (300MHz, DMSO-d 6 )δ (ppm) 1 65.4 1 55.9 1 54.2 1 54.0 1 53.8 153.4 140.9 138.6 134.3 130.6 124.9 123.2 120.9 112.1 69.3 65.6 62.853.5 39.3 14.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com