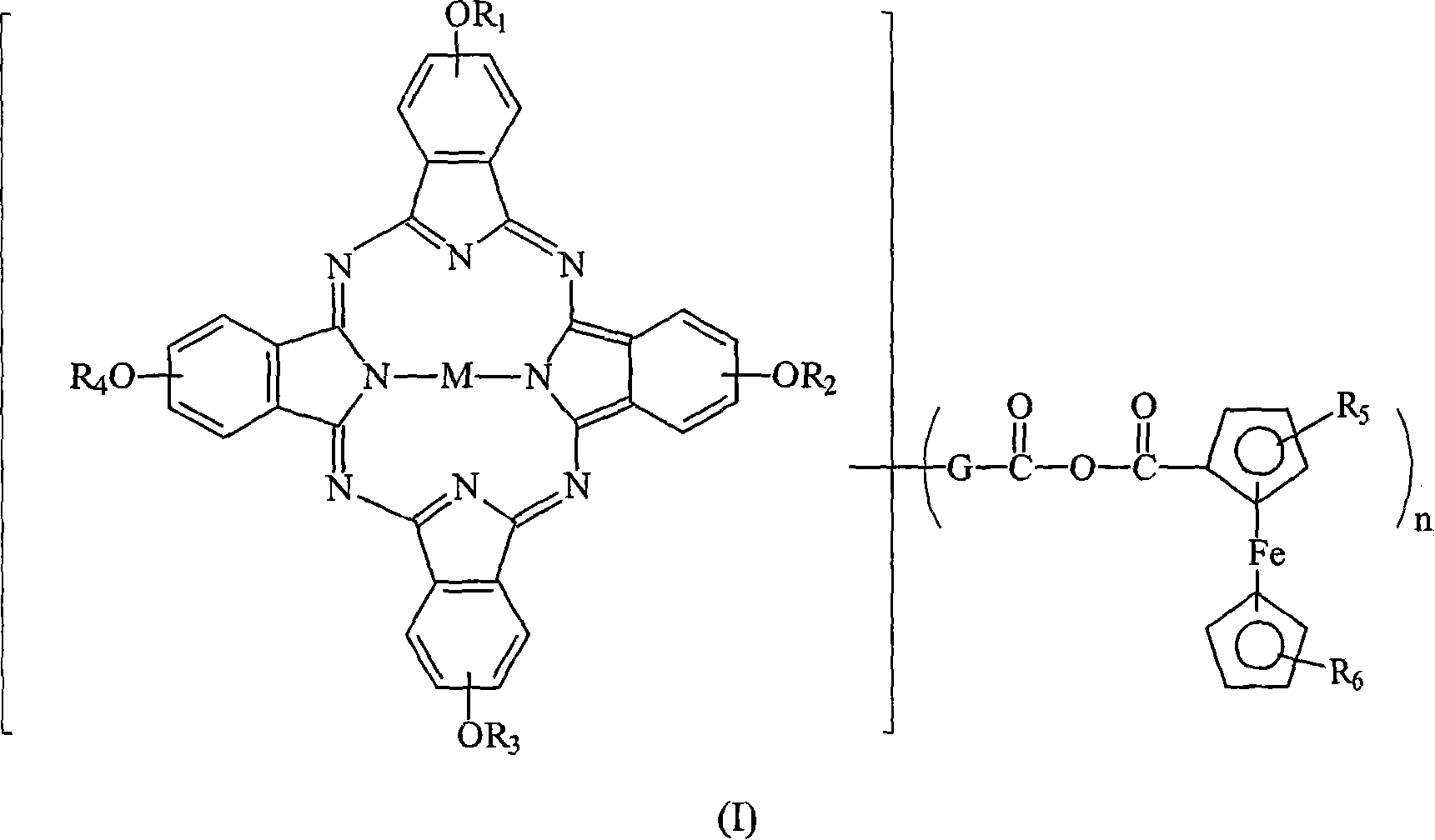
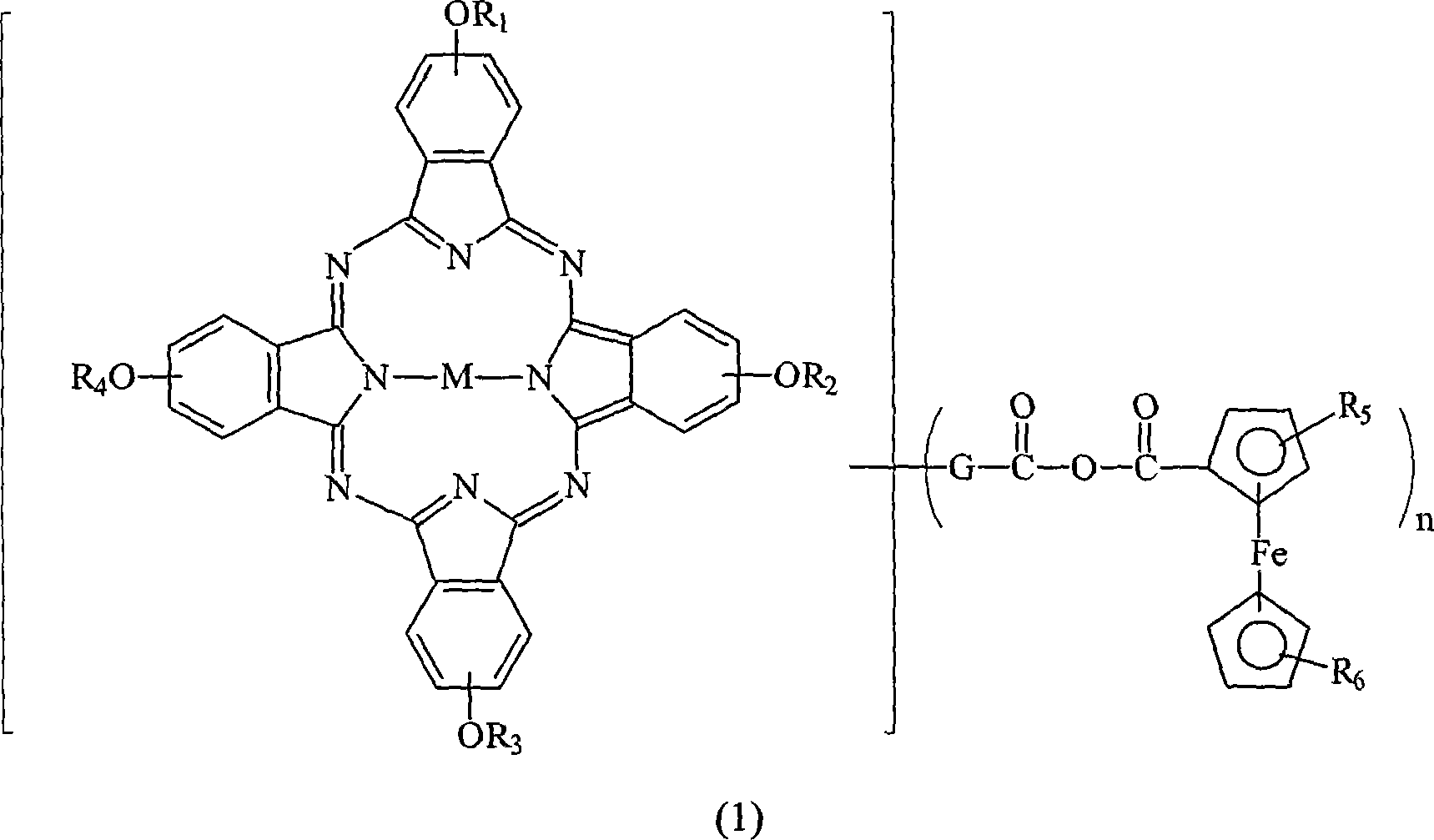
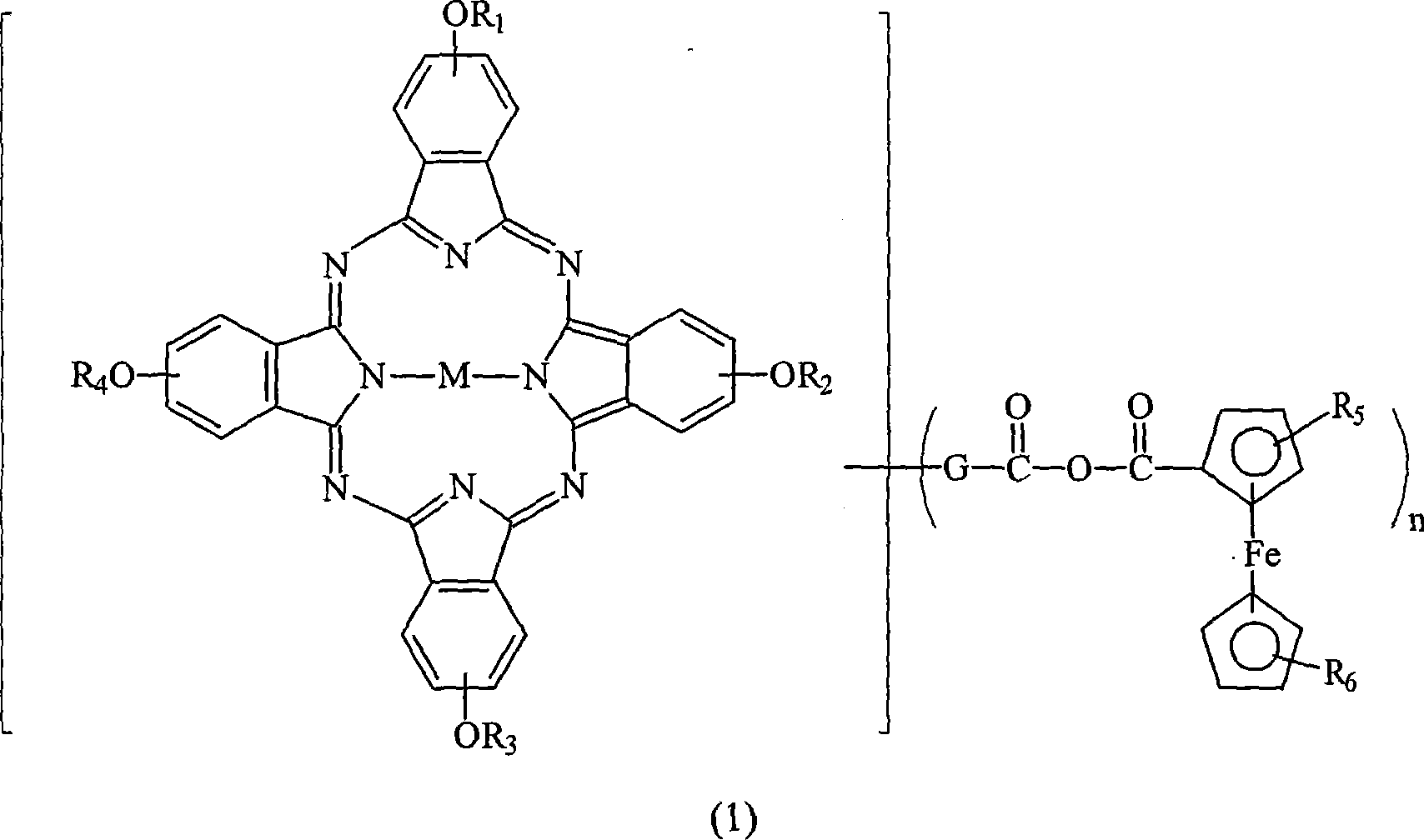
Optical dye of phthalocyanine derivative, and application in recording medium
A technology of optical recording and derivatives, applied in optical, electrical recording, azo dyes, etc., can solve the problems of etch pit offset, inability to take into account low speed, inaccurate control of signal etch pit length, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Weigh 10.0 grams of four-alpha (2,4-dimethyl-3-pentyloxy) copper phthalocyanine derivatives (prepared according to EP 703280) and add to a 250 ml round-bottom bottle filled with nitrogen, then add 50 ml of After dissolving toluene and 5.4 grams of N-methylformamide, the temperature of the solution was lowered to 0°C, and then 5.6 grams of phosphorus oxychloride (POCl 3 ) was slowly added to the reaction solution, and the rate of addition was controlled to maintain the temperature of the reaction solution not exceeding 5°C. After the addition was complete, the cooling system was removed and the temperature of the reaction solution was raised to 50°C. The reaction solution was stirred at 50°C for 24 hours and the progress of the reaction was monitored by TLC. After the reaction, the reaction solution was poured into 200 ml of sodium acetate (41.5 g) ice-water mixture, the mixture was stirred for 30 minutes, and then the mixture was extracted with 100 ml×3 toluene. Collec...
Embodiment 2
[0052] Weigh 1.03 g of sodium borohydride (sodium borohydride) and add it to a 250 ml round-bottomed three-neck flask filled with nitrogen, then add 40 ml of ethanol and stir to mix it to make most of it dissolve. 10.0 grams of formylated tetra-α-(2,4-dimethyl-3-pentyloxy) copper phthalocyanine derivatives (prepared in Example 1) were dissolved in 40 milliliters of THF solution, and then added to the above reduction Among the reagents, the reaction solution was stirred vigorously at room temperature for 24 hours and the progress of the reaction was monitored by thin layer chromatography. After the reaction, the reaction mixture was filtered to remove insoluble matter, and poured into 200 ml of 20% saline to terminate the reaction, and then the mixture was extracted with 40 ml×3 toluene. Collect the organic layer uniformly, add 20 grams of anhydrous magnesium sulfate to dry it, filter to remove hydrous magnesium sulfate, concentrate under reduced pressure to 40 ml, pour the con...
Embodiment 3
[0057] Into a 500 ml reaction flask filled with nitrogen, 4.18 g of ferrocenecarboxylic acid and 20 ml of dichloromethane were added. At a low temperature of 0-5°C, 2.43 g of oxalyl chloride was slowly added thereto. After 1 hour, excess (or unreacted) oxalyl chloride was stripped off under reduced pressure. After that, 25 mL of pyridine was added slowly and the reaction temperature was controlled below 15 °C. Separately, 10 g of the compound of Example 2 was dissolved in 22.5 ml of dichloromethane, and this solution was added to the previous 500 ml reaction bottle and allowed to react for 3 hours. Finally, the reactants were poured into methanol / water (75 / 25) mixed solution to terminate the reaction. The formed green powder was filtered, and the product was dried under vacuum at 70° C. for two days to obtain 9.7 g (78% of theory) of a green powder product.
[0058] UV-VIS (DBE): λmax = 712 nm.
[0059] IR (KBr): 1715, 1743, 1770cm -1 .
[0060] TGA: Major decomposition ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com