Novel peacock blue derivative, its synthesis and uses in recording medium
A derivative, phthalocyanine technology, applied in the application field of disposable recording discs, can solve the problems of high synthesis difficulty, inability to meet the urgent needs of cheap and high-quality products, and expensive raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
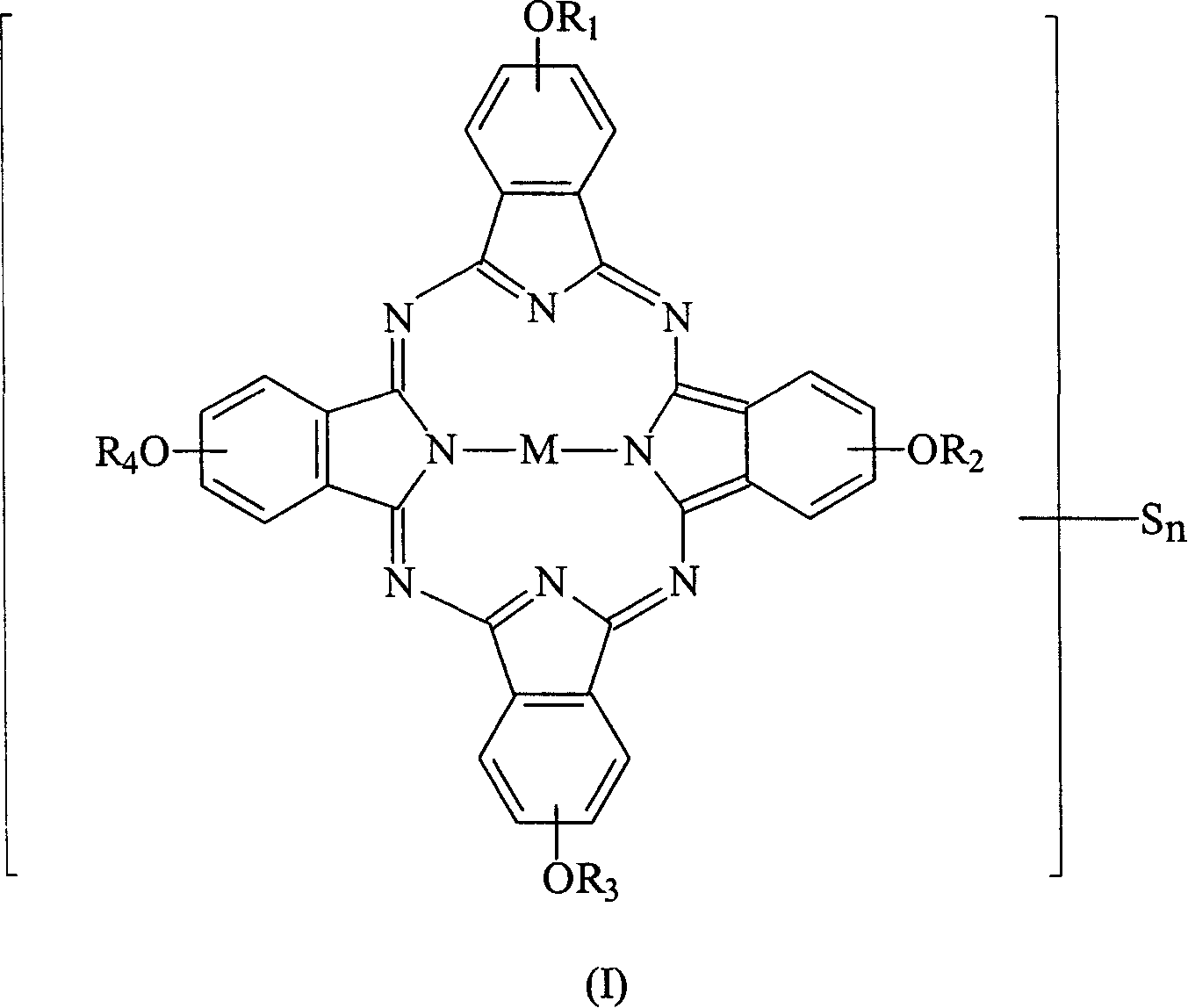
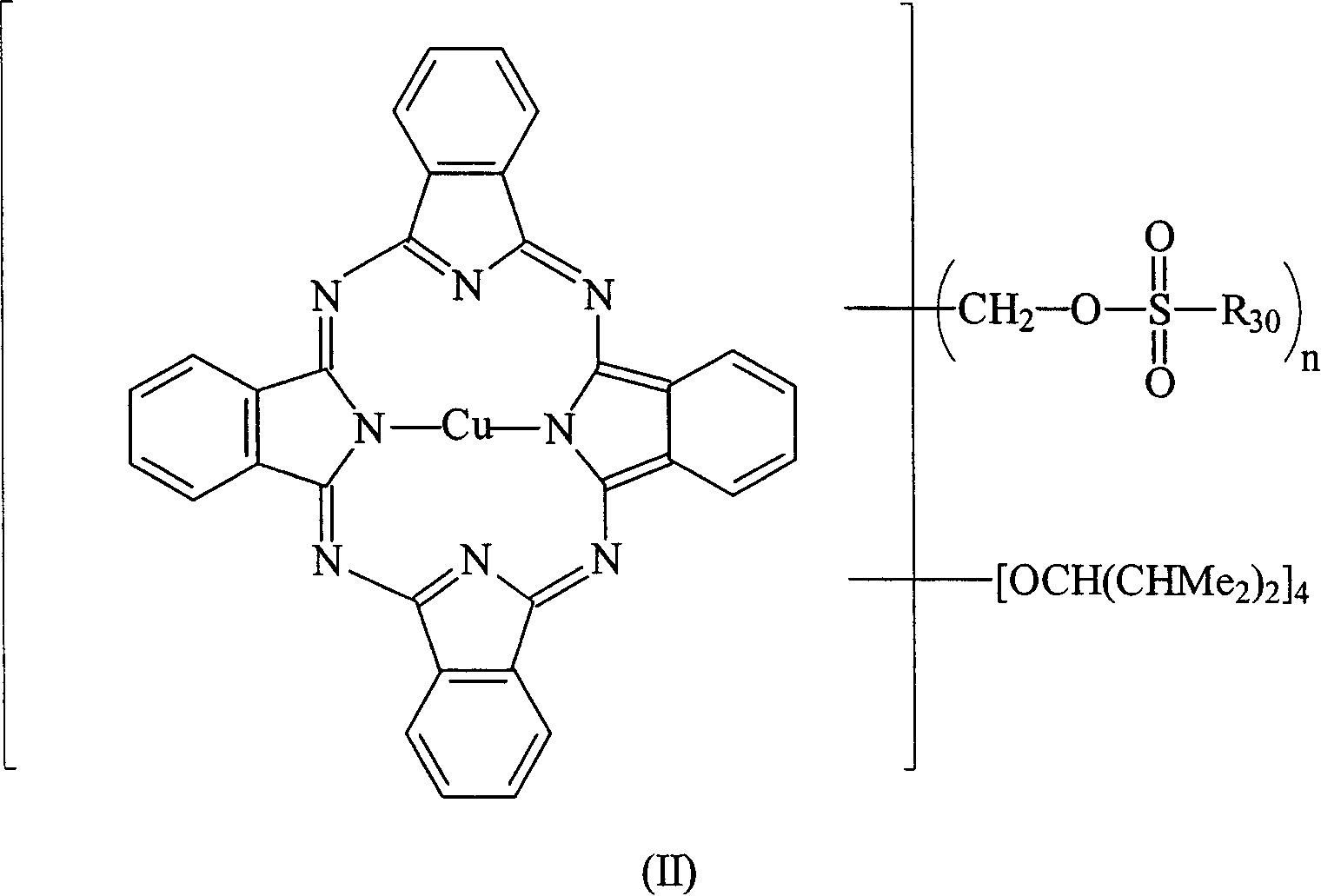
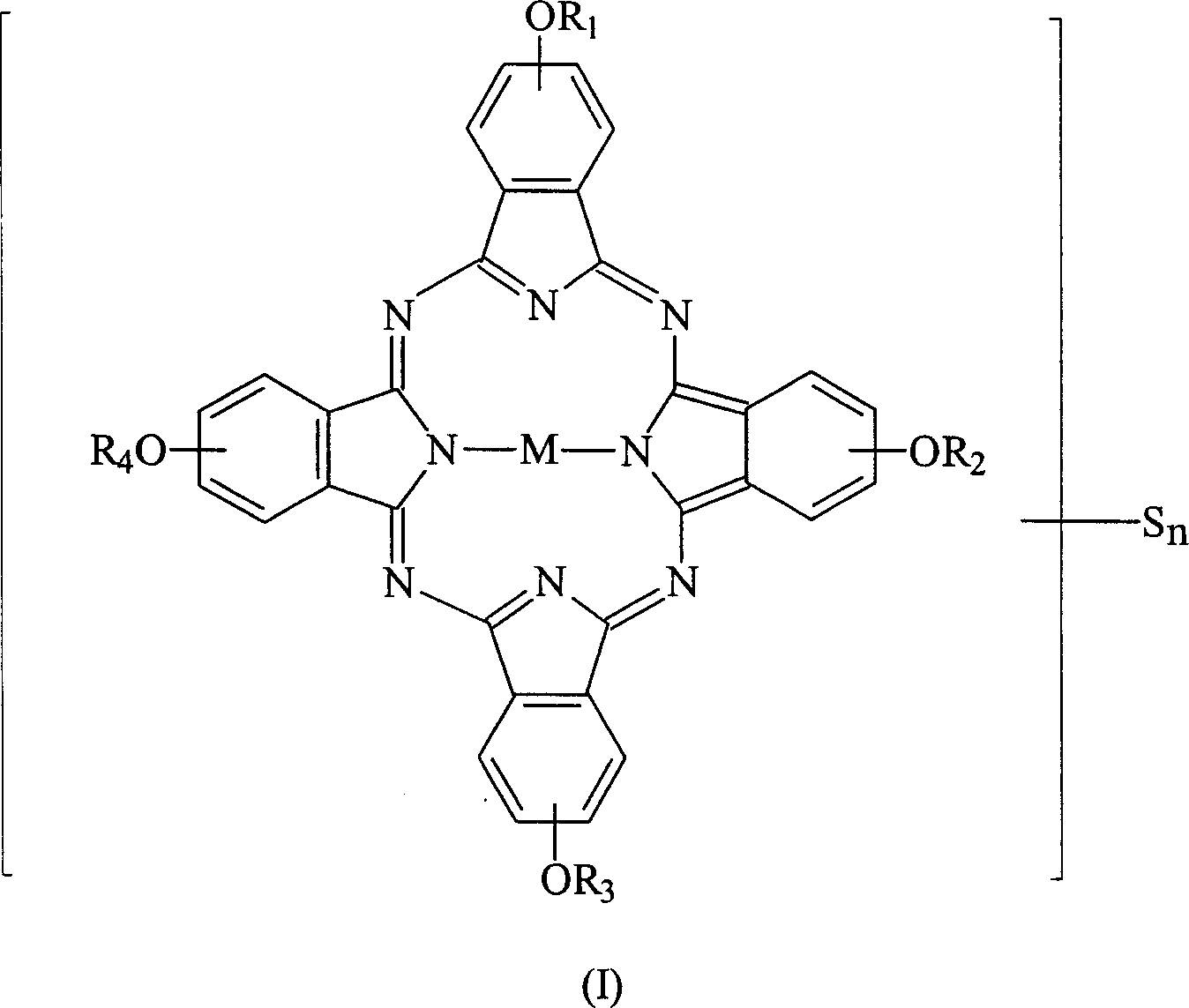
[0061] Weigh 10.0 g of tetra-α-(2,4-dimethyl-3-pentyloxy) copper phthalocyanine derivative (prepared according to EP 703280) into a 250 ml round bottom bottle filled with nitrogen, then add 50 ml of toluene and 5.4 grams of N-methylformamide, after they were dissolved, the temperature of the solution was lowered to 0°C, and then 5.6 grams of phosphorus oxychloride (POCl 3 ) was slowly added to the reaction solution, and the rate of addition was controlled to maintain the temperature of the reaction solution not exceeding 5°C. After the addition was complete, the cooling system was removed and the temperature of the reaction solution was raised to 50°C. The reaction solution was stirred at 50°C for 24 hours and the progress of the reaction was monitored by TLC. After the reaction, the reaction solution was poured into 200 ml of sodium acetate (41.5 g) ice-water mixture, the mixture was stirred for 30 minutes, and then the mixture was extracted with 100 ml×3 toluene. Collect th...
Embodiment 2
[0067] Weigh 1.03 g of sodium borohydride and add it to a 250 ml round-bottomed three-necked flask filled with nitrogen, then add 40 ml of ethanol and stir to mix to dissolve most of it. 10.0 grams of formylated tetra-α-(2,4-dimethyl-3-pentyloxy) copper phthalocyanine derivatives (prepared in Example 1) were dissolved in 40 milliliters of THF solution, and then added to the reducing agent In solution, the reaction solution was stirred vigorously at room temperature for 24 hours and the progress of the reaction was monitored by thin layer chromatography (TLC). After the reaction, the reaction mixture was filtered to remove insoluble matter, and poured into 200 ml of 20% saline to terminate the reaction, and then the mixture was extracted with 40 ml×3 toluene. Collect the organic layer uniformly, add 20 grams of anhydrous magnesium sulfate to dry, filter to remove hydrous magnesium sulfate, concentrate under reduced pressure to 40 ml, pour the concentrated solution into 1 liter ...
Embodiment 3
[0073] Weigh 10.0 grams of hydroxymethylated tetra-α-(2,4-dimethyl-3-pentyloxy) copper phthalocyanine derivatives (prepared in Example 2) in a 250 ml reactor, and then Add 40 ml of toluene and stir. After the solution was dissolved, the temperature of the solution was lowered to 0° C., and then 48.64 ml of 6.25% trifluoromethanesulfonic anhydride (trifluoromethanesulfonic anhydride) toluene solution was slowly added to the reaction solution, and the temperature of the reaction solution was kept below 5° C. After the addition was complete, the cooling system was removed to allow the temperature of the reaction solution to return to room temperature, and the reaction solution was stirred at room temperature for 2 hours, and the progress of the reaction was monitored by TLC. After the reaction was over, the reaction solution was poured into 80 / 240 ml of methanol / water mixture to terminate the reaction, then the mixture was stirred for 30 minutes, then the mixture was extracted wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com