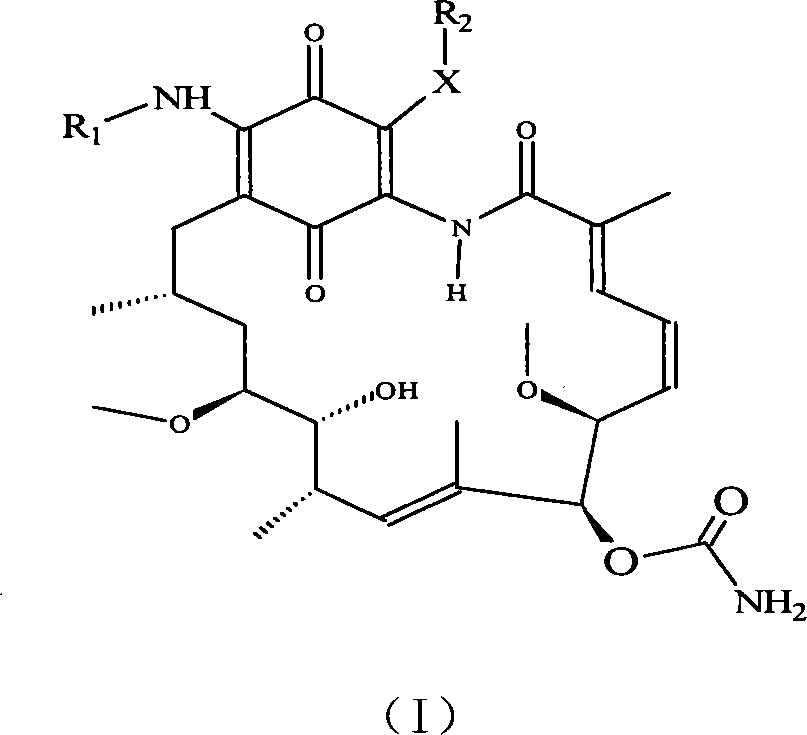
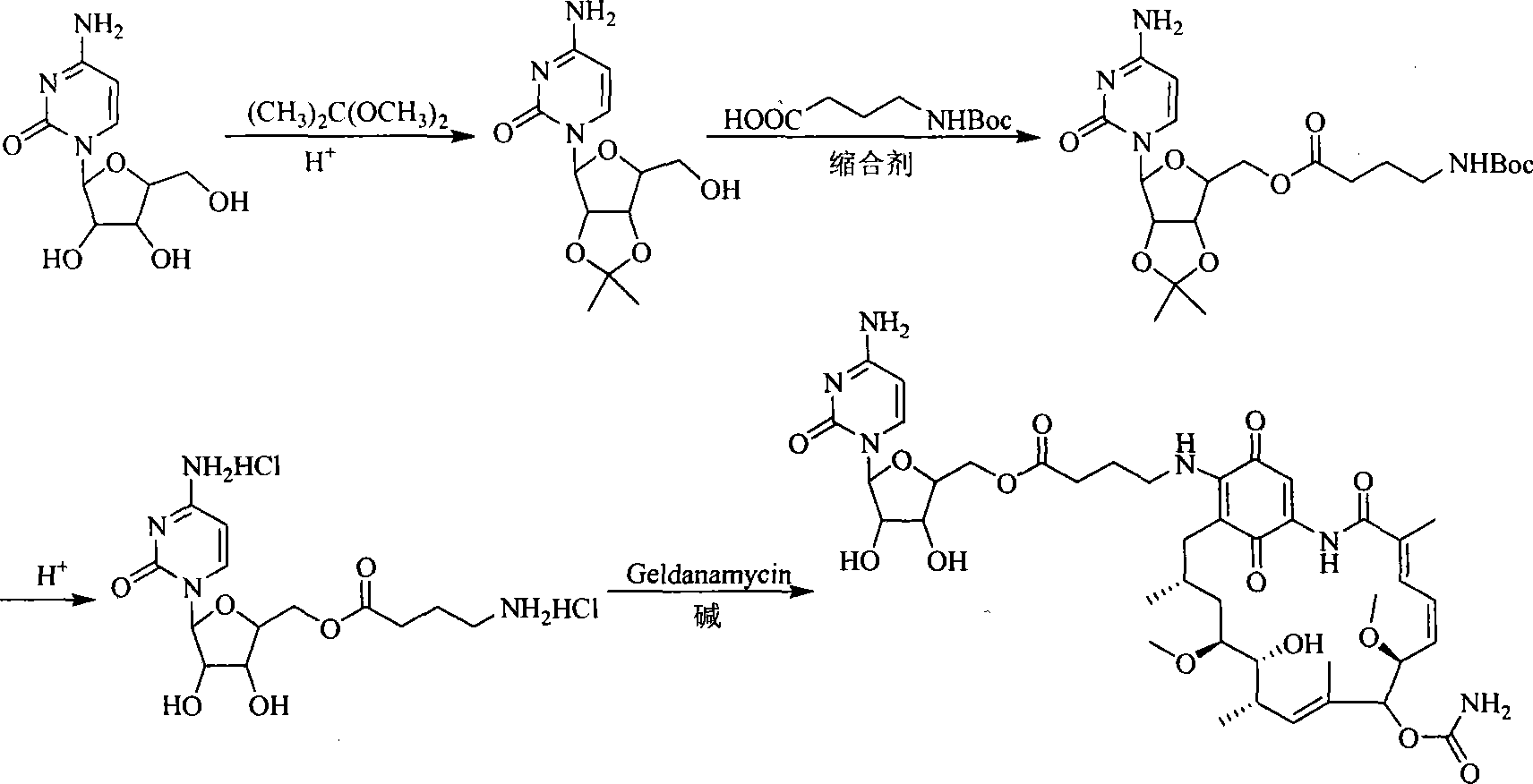
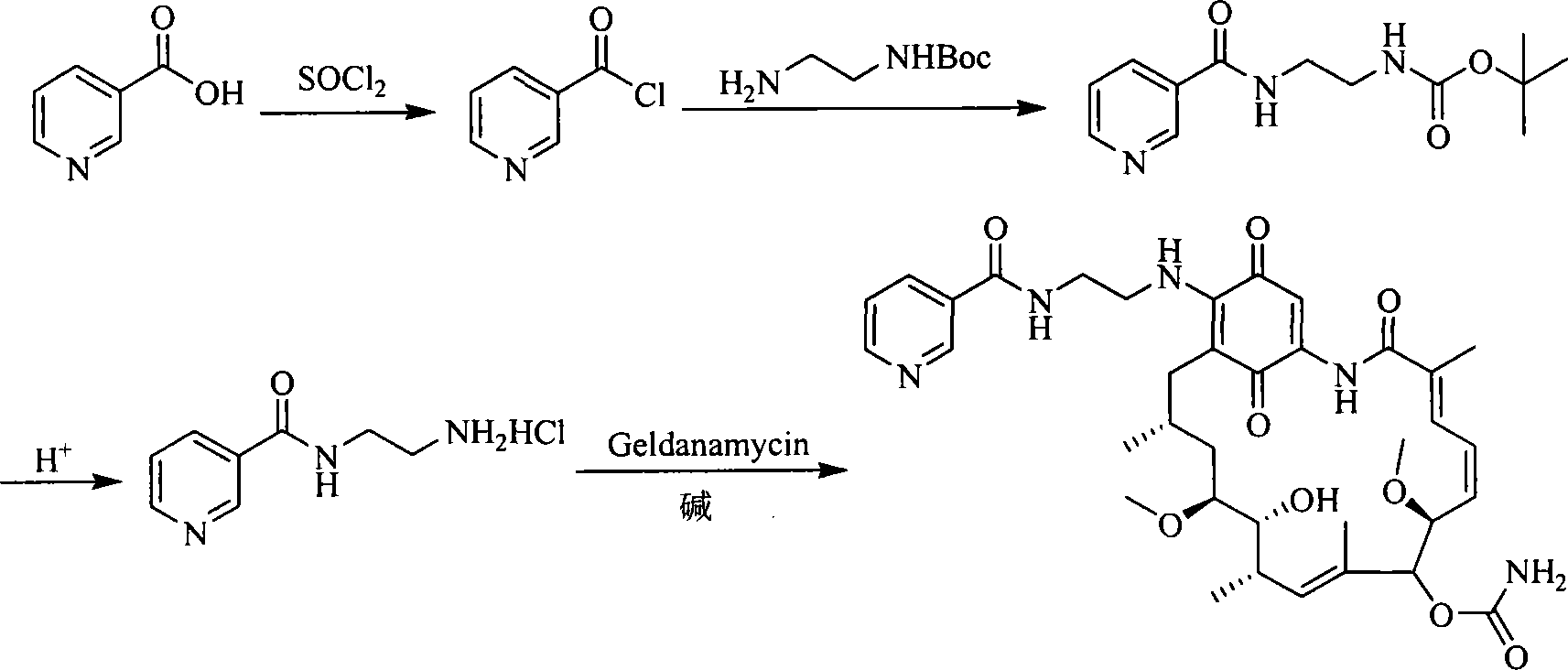
A set of geldanamycin derivant and method for preparing the same
A technology of geldanamycin and derivatives, applied in the field of geldanamycin structure modification derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 >: Preparation of 17-(2'-(1",4"-oxazepinyl-1"-)ethylamino)-17-desmethoxygeldanamycin GM-APML):
[0044] Take geldanamycin 50mg (89.29μmol), add 5mL CHCl 3 , methanol 0.5ml, stirred until the geldanamycin was dissolved, and the reaction solution was orange-yellow. Add 21 mg (164 μmol) of 4-(2-aminoethyl)-1,4-oxazepine, react at room temperature for 4 days, evaporate the solvent to obtain a dark purple solid, and dissolve the solid residue in 10 mL of ethyl acetate , followed by deionized water, saturated NaHCO 3 , 1mol / L HCl solution, washed with saturated brine. Anhydrous Na was added to the organic phase 2 SO 4 Let dry overnight. The desiccant was filtered off, and the organic phase was concentrated under reduced pressure. Chromatographic separation was performed on a silica gel column to obtain 46.2 mg (61.2%) of the compound GM-APML.
[0045]1 H-NMR (400MHz, CDCl 3 )δ(ppm): 0.9~1.0(m, 6H, C10-CH3, C14-CH3), 1.28-1.38(m, 2H, C13-H2), 1.5(m, 1H, C14-H...
Embodiment 2
[0046] Example 2 >: Preparation of 17-(2'-(1"-azacyclohexyl-1"-)ethylamino)-17-desmethoxygeldanamycin (GM-AEPD):
[0047] When the side chain reactant is 2-(1'-azacyclohexyl)ethylamine, the compound GM-AEPD is synthesized according to the method similar to Example 1.
[0048] 1 H-NMR (400MHz, CDCl 3 )δ(ppm): 0.82(1H, m, C14-H), 0.94~1.0(m, 6H, C10-CH3, C14-CH3), 1.24-1.3(m, 4H, C13-H2, C15-H2) , 1.4~1.5(m, 2H, C17-N(CH2-CH2) 2 CH2 )), 1.6(br, 4H, C17-N(CH2- CH2 ) 2 CH2), 1.76(br, 1H, C10-H), 1.78(s, 3H, C8-CH3), 2.03(s, 3H, C2-CH3), 2.3~2.4(br, 4H, C17-N-(C H2 -CH2) 2 CH2), 2.6~2.8(m, 4H, C17-NH- CH2-CH2 -N), 3.24(s, 3H, C12-OCH3), 3.38(s, 3H, C6-OCH3), 3.44(d, 1H, J=9.2Hz, C12-H), 3.58(d, 1H, J= 9.2Hz, C11-H), 3.7(br, 1H, C17-NH-), 4.31(d, 1H, J=10.0Hz, C6-H), 4.5(br, 1H, C11-OH), 4.80(br , 2H, -CO-NH2), 5.20 (s, 1H, C7-H), 5.83 (t, 1H, J=10.4, C5-H) 5.94 (d, 1H, J=9.6, C9-H), 6.59 (t, 1H, J=11.6Hz, C4-H), 6.96(d, 1H, J=11.6Hz, C3-H), 7.22(br, 1H, C20-NH-CO), 9...
Embodiment 3
[0049] Example 3 >: 1 Preparation of 7-(4'-benzyl-4'-azacyclohexylamino)-17-desmethoxygeldanamycin (GM-ABPD):
[0050] When the side chain reactant was 4-benzyl-4-azacyclohexylamine, the compound GM-ABPD was synthesized according to the method similar to Example 1.
[0051] 1 H-NMR (400MHz, CDCl 3 )δ(ppm): 0.94~1.0(dd, 6H, C10-CH3, C14-CH3), 1.5~1.6(m, 4H, C17-NH-CH( CH2 -CH2) 2 N-), 1.64(d, 2H, C15-H2), 1.7(m, 2H, C13-H2), 1.8(s, 3H, C8-CH3), 1.9(s, 2H, C17-NH-CH-( CH2-CH2) 2 -N- CH2 -Ph), 2.03(s, 3H, C2-CH3), 2.1-2.2(m, 2H, C17-NH-CH-(CH2- CH2 ) 2 -N-CH2-Ph), 2.7~2.8(m, 3H, C14-CH, C17-NH-CH-(CH2- CH2 ) 2 -N-CH2-Ph), 2.87(br, 1H, C17-NH-C H (CH2-CH2) 2 N-), 3.26(s, 3H, C12-OCH3), 3.38(s, 3H, C6-OCH3), 3.4(d, 1H, J=9.2Hz, C12-H), 3.58(d, 1H, J= 9.2Hz, C11-H), 3.6(s, 1H, C10-H), 3.9(br, 1H, C17-NH-), 4.2(br, 1H, C11-OH), 4.3(d, 1H, J= 10.0Hz, C6-H), 4.78(br, 2H, -CO-NH2), 5.17(s, 1H, C7-H), 5.8-5.9(m, 2H, C5-H, C9-H), 6.27( br, 1H, C20-NH-CO), 6.5 (t, 1H, J=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com