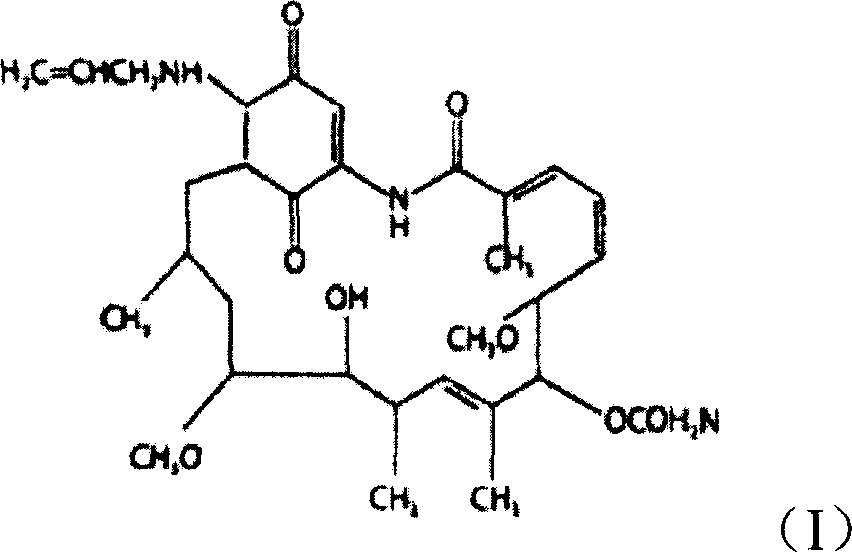
Injection use medicine composition contg. water-soluble 17-allylamino-17-demethoxy geldanamycin
A technology of demethoxygeldanamycin and allylamino, which is applied in the field of pharmaceutical compositions for injection of 17-allylamino-17-demethoxygeldanamycin, can solve the problem of large side effects, Limitation, lack of tumor cell specificity and other problems, to achieve the effect of drug safety and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] 1. Establishment of HPLC determination method for 17-AAG influencing factors test
[0097] Use octadecylsilane bonded silica gel as filler; phosphate buffer [take sodium dihydrogen phosphate (NaH 2 PO 4 2H 2 (0) 2g, dissolved in water and diluted to make 1000ml]-acetonitrile-methanol-triethylamine (40:50:10:0.2) is mobile phase adjusted to pH 5.0 with phosphoric acid; flow velocity is 1.2ml / min; detection wavelength is 303nm . The number of theoretical plates based on 17-AAG should not be less than 2000; the separation between 17-AAG peak and adjacent impurity peak should meet the requirements.
[0098] 2. Test methods and results
[0099] 1. Sample control test
[0100] Avoid light operation. Take about 15 mg of 17-AAG, weigh it accurately, put it in a 50ml measuring bottle, add an appropriate amount of mobile phase, and treat it ultrasonically for 5 minutes. 3 times of the retention time of the component peak, the total impurity is 1.26% according to the normal...
Embodiment 2
[0118] Taking different ratios of 17-AAG and excipient PEG400, the interaction between raw materials and excipients was investigated under the influence of light and temperature. Use HPLC method to check the content of 17-AAG and the changes of related substances before and after being placed, and observe the changes of drug properties such as appearance and color at the same time, so as to evaluate the compatibility of raw materials and excipients.
[0119] 1. HPLC detection method for related substances in 17-AAG preparations
[0120] Use octadecylsilane bonded silica gel as filler; phosphoric acid aqueous solution (use phosphoric acid to adjust the pH value to 3.0) as mobile phase A, and acetonitrile as mobile phase B; flow rate is 1.0ml per minute, gradient elution; detection wavelength 303nm. The number of theoretical plates based on 17-AAG should not be less than 2000; the separation between 17-AAG peak and adjacent impurity peak should meet the requirements. This HPLC...
Embodiment 3
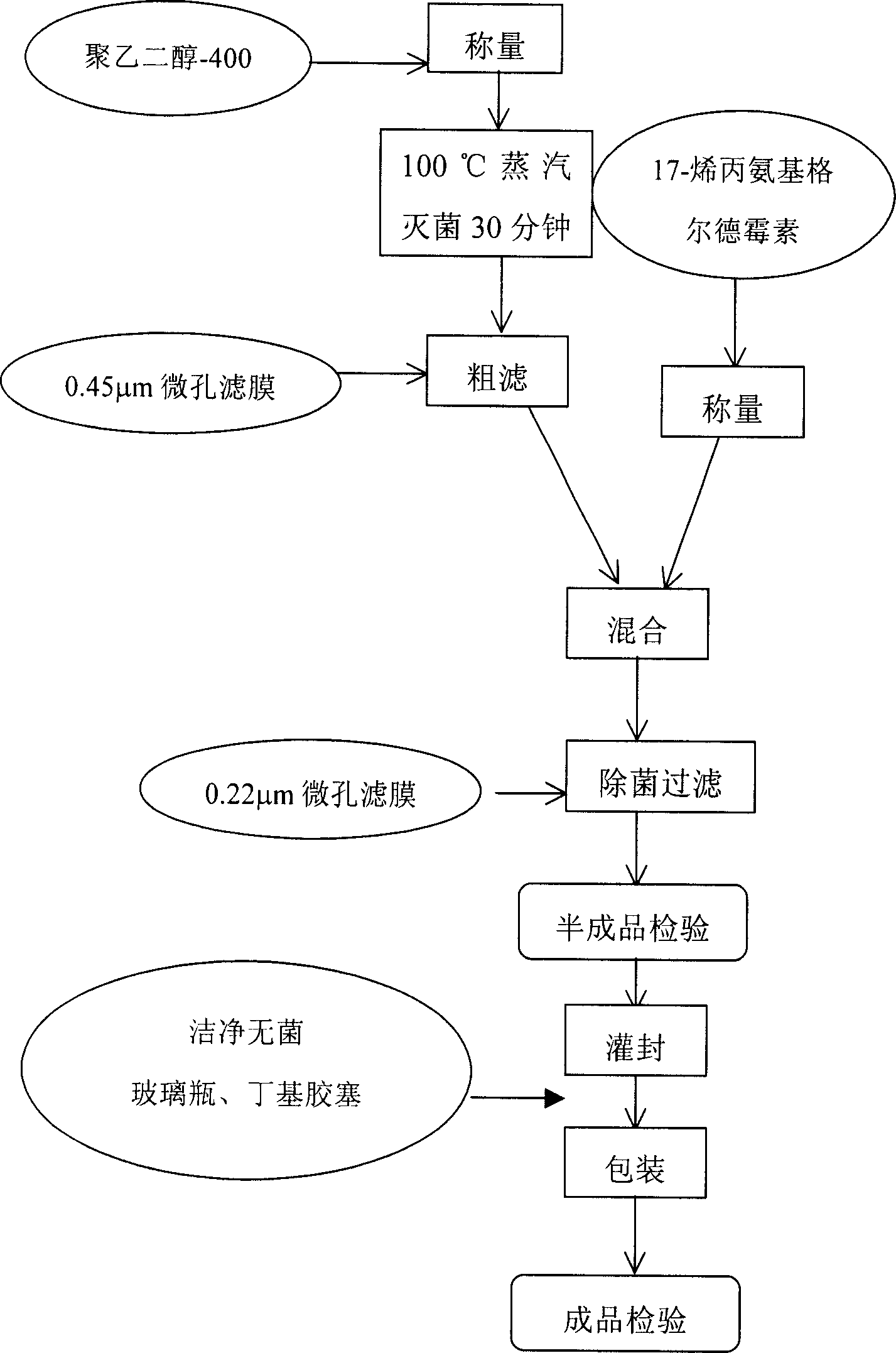
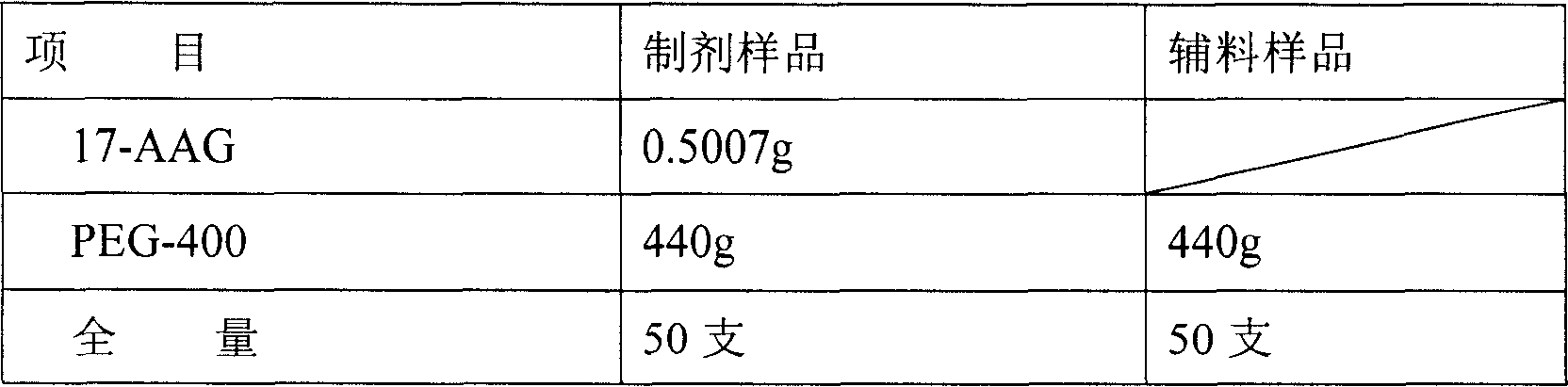
[0136] Accurately weigh 1000 mg of 17-AAG, add it into 800 ml of PEG400 (Shanghai Pudong Gaonan Chemical Factory, batch number 040448) solution and dissolve. Sterilize and filter at room temperature in the dark. The preparation was dispensed into sterilized vials under aseptic conditions.
[0137] Add it to 0.9% sodium chloride injection or 5% or 10% glucose saline injection before clinical application to make the final concentration of PEG400 reach 30%-40% or 10%-30%. The 17- AAG remained stable within 4 hours.
[0138] For the process flow of 17-AAG injection preparation, see figure 1 .
[0139] The preparation formula of the present invention can obviously improve the water solubility of 17-AAG, and the result shows that the water solubility of 17-AAG can be increased by more than 50 times.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com