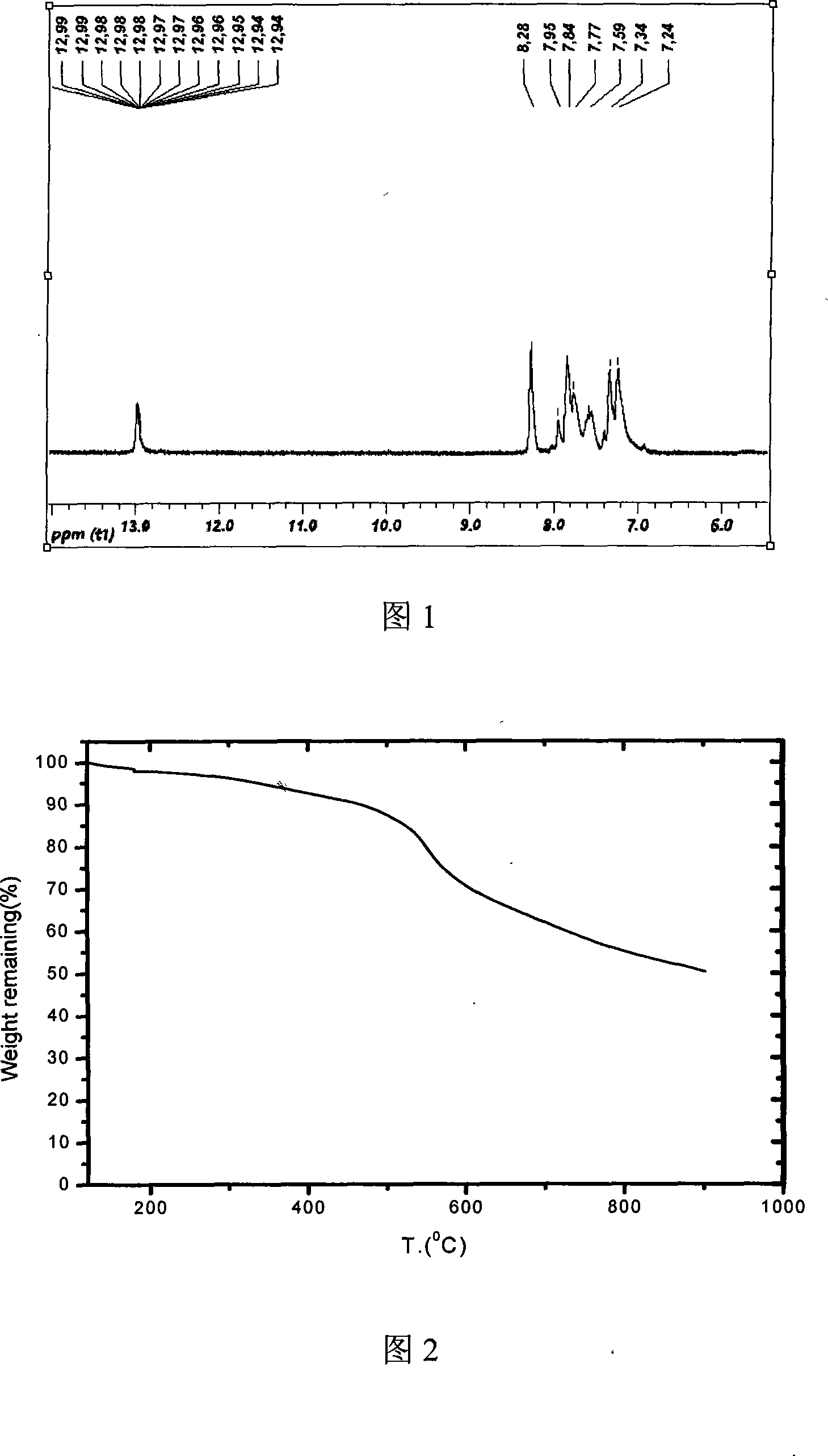
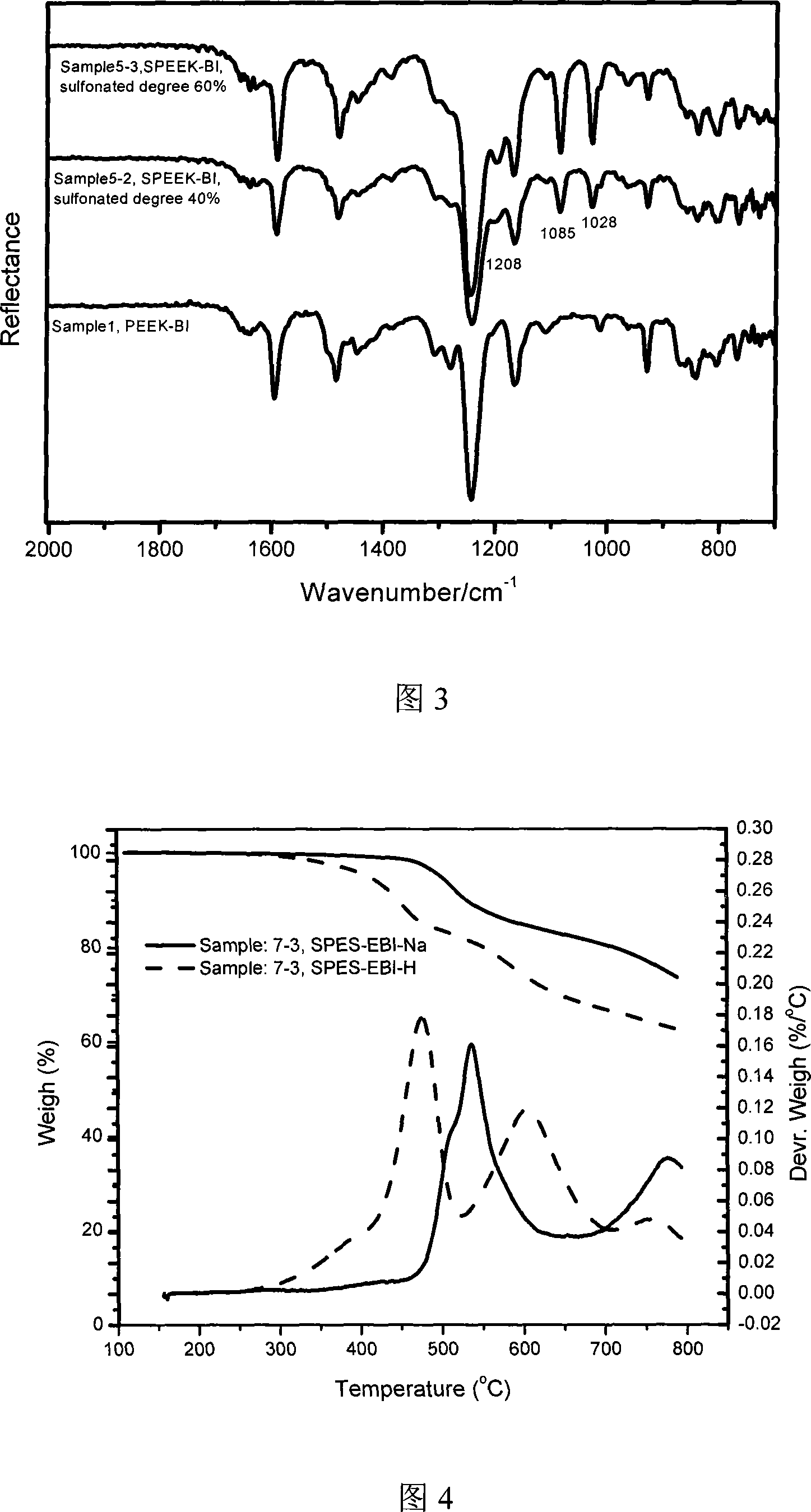
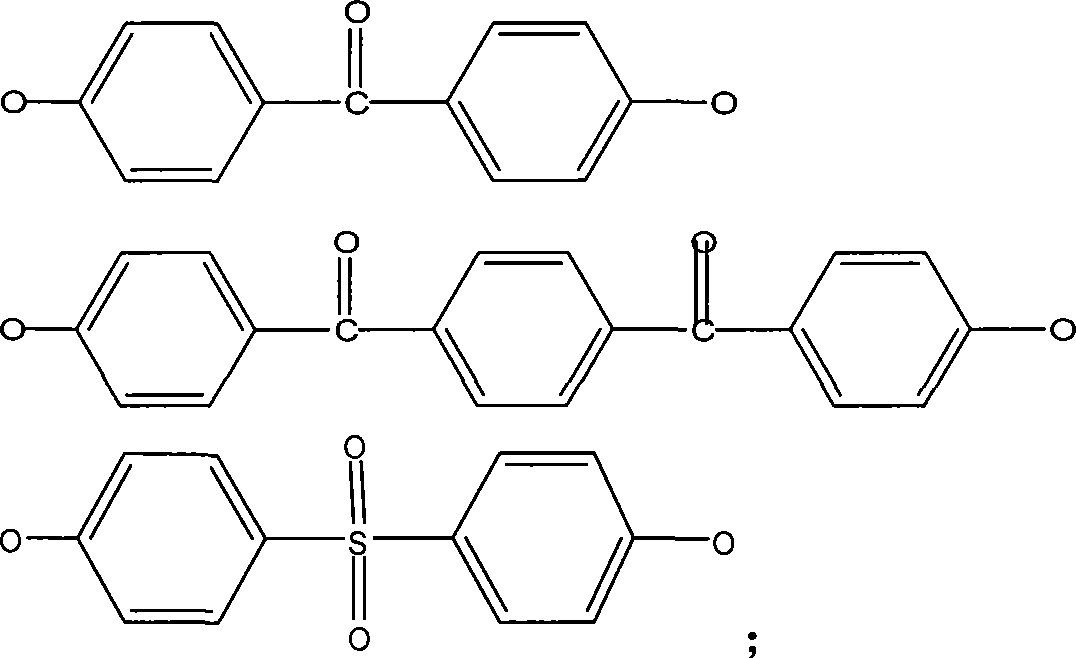
Polybenzimidazole containing ether ketone/ether sulfone structure as well as sulfonated polymer and preparation method thereof
A technology of polybenzimidazole sulfonate and polybenzimidazole is applied in the field of polybenzimidazole containing ether ketone structure or ether sulfone structure and sulfonated polymers thereof, and can solve the problems of degradation, cross-linking side reaction, and difficulty in sulfonation degree. Accurate control and other issues to achieve the effect of improving solubility, excellent electrical properties, and improving alcohol resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Add 30mL dimethyl sulfoxide (DMSO) and 15mL toluene to a three-necked flask equipped with a water separator, a condenser and a nitrogen pipe. 2 Reflux at 140°C for 3h under protection, distill off the toluene and cool down to room temperature. Weigh 0.8369g (2mmol) of 5,5'-bis(2-p-hydroxyphenyl-benzimidazole) monomer, 0.4364g (2mmol, 1 equivalent) of 4,4'-difluorobenzophenone, K 2 CO 3 Add 0.5528g (4mmol) into a three-necked flask filled with DMSO, and stir to obtain a light yellow transparent solution with a small amount of insoluble matter at the bottom. in N 2 Keep the temperature at 145°C for 8h under protection and magnetic stirring. When the temperature was adjusted to 120°C, the reaction solution turned brown and opaque. After 1 hour, add 10ml of toluene, raise the temperature to 150°C, and reflux for 2 hours. Water will precipitate in the water separator. After the toluene is evaporated, keep the temperature at 160°C for 14 hours. Cool the reaction solution...
Embodiment 2
[0058] Add 30mL dimethyl sulfoxide (DMSO) and 15mL toluene to a three-necked flask equipped with a water separator, a condenser and a nitrogen pipe. 2 Reflux at 140°C for 3h under protection, then distill off the toluene and cool down to room temperature.
[0059] Weigh 0.8689g (2mmol) of 5,5'-bis(2-p-hydroxyphenyl-benzimidazolium)-ether monomer, 0.4364g (2mmol) of 4,4'-difluorobenzophenone, K 2 CO 3 Add 0.5528g (4mmol) into a three-necked flask filled with DMSO, and stir to obtain a light yellow transparent solution with a small amount of insoluble matter at the bottom. in N 2 Under protection and magnetic stirring, keep the temperature at 260°C for 8 hours, then add 10ml of toluene, raise the temperature to 150°C, reflux for 2 hours, divide the water with the water separator, and then steam the toluene and keep the temperature at 160°C for 14 hours. Cool the reaction liquid to 80°C, pour it into deionized water under stirring, and produce a large amount of flocculent pre...
example 3
[0063] Add 30mL of dimethylformamide (DMF) and 15mL of water-carrying agent toluene into a three-neck flask equipped with a water separator, a condenser and a nitrogen pipe, and 2 Reflux at 140°C for 3h under protection, then distill off the toluene and cool down to room temperature.
[0064] Weigh 0.4344 g (1 mmol) of 5,5'-bis(2-p-hydroxyphenyl-benzimidazole)-ether monomer and 5,5'-bis(2-p-hydroxyphenyl-benzimidazole) mono Body 0.4184g (1mmol), 4,4'-difluorobenzophenone 0.4364g (2mmol), K 2 CO 3 Add 0.5528g (4mmol) into a three-necked flask filled with DMF, and stir to obtain a light yellow transparent solution with a small amount of insoluble matter at the bottom. in N 2 Under protection and magnetic stirring, keep the temperature at 135°C for 8 hours, then add 10ml of toluene, raise the temperature to 190°C, reflux for 2 hours, divide the water with a water separator, and then evaporate the toluene and keep the temperature at 140°C for 12 hours. Cool the reaction soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com