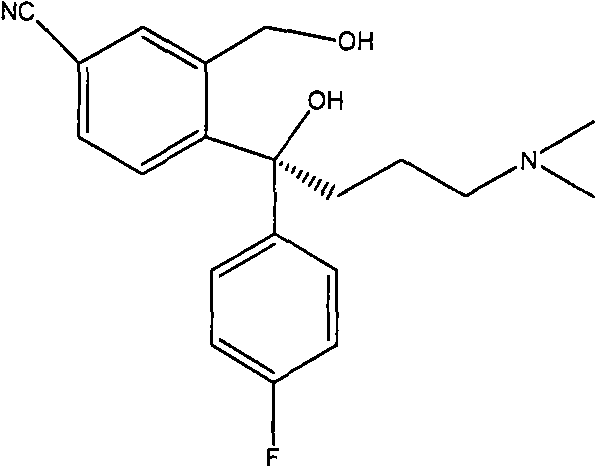
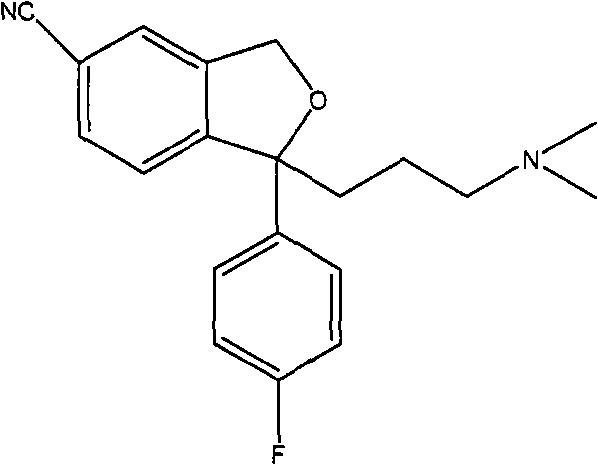
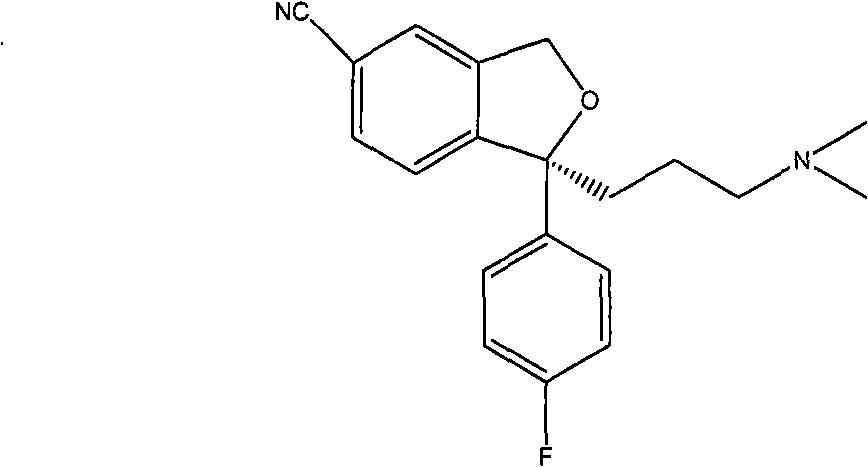
Method for preparing (S)-citalopram intermediate S-type glycol
A technology of dextrocitalopram, type diol, applied in the directions of organic chemistry methods, chemical instruments and methods, organic racemization, etc., can solve the problems of high cost, poor stability and reproducibility, great difficulty, etc. Simple, stable and reproducible results at low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 55.1g 4-[4-(dimethylamino)-1-(4'-fluorophenyl)-1-hydroxybutyl]-3-(hydroxymethyl)-cyanobenzene in 100ml dichloromethane solution and D - Mix 32.5 g of p-methyldibenzoyl tartaric acid in 500 ml of absolute ethanol and heat until the solids are completely dissolved. Slowly lowered to room temperature, then lowered to 0°C, stirred for 1h. Filter and wash with a small amount of dichloromethane: absolute ethanol (1:5). dry. 32.1 grams of 4-[4-(dimethylamino)-1-(4'-fluorophenyl)-1-hydroxybutyl]-3-(hydroxymethyl)-cyanobenzene.1 / 2DTTA salt was obtained, yield 36.7%.
[0040] The above solid was dissolved in 400 ml of a mixed solvent of dichloromethane and absolute ethanol (ratio 1:5) for recrystallization to obtain 29.1 g of solid, with a yield of 93%.
[0041] After detection: the total yield of resolution: 34.1%; the optical degree is 99.4% (HPLC).
Embodiment 2
[0043] 55.1g 4-[4-(dimethylamino)-1-(4'-fluorophenyl)-1-hydroxybutyl]-3-(hydroxymethyl)-cyanobenzene in 150ml dichloromethane solution and D - Mix 32.5 g of p-methyldibenzoyl tartaric acid in 350 ml of absolute ethanol and heat until the solids are completely dissolved. Slowly lowered to room temperature, then lowered to 0°C, stirred for 1h. Filter and wash with a small amount of dichloromethane: absolute ethanol (3:5). dry. 34.5 grams of 4-[4-(dimethylamino)-1-(4'-fluorophenyl)-1-hydroxybutyl]-3-(hydroxymethyl)-cyanobenzene.1 / 2DTTA salt was obtained, yield 39.4%.
[0044] The above solid was dissolved in 350 ml of a mixed solvent of dichloromethane and absolute ethanol (ratio 3:5) for recrystallization to obtain 32.8 g of solid, with a yield of 95%.
[0045] After detection: the total yield of resolution: 37.4%; the optical degree is 99.6% (HPLC).
Embodiment 3
[0047] 55.1g of 4-[4-(dimethylamino)-1-(4'-fluorophenyl)-1-hydroxybutyl]-3-(hydroxymethyl)-cyanobenzene in 450ml of dichloromethane solution and D - Mix 32.5 g of p-methyldibenzoyl tartaric acid in 150 ml of absolute ethanol and heat until the solids are completely dissolved. Slowly lowered to room temperature, then lowered to 0°C, stirred for 1h. Filter and wash with a small amount of dichloromethane: absolute ethanol (3:1). dry. 40.5 g of 4-[4-(dimethylamino)-1-(4'-fluorophenyl)-1-hydroxybutyl]-3-(hydroxymethyl)-cyanobenzene.1 / 2DTTA salt was obtained, yield 46.3%.
[0048] The above solid was dissolved in 350 ml of a mixed solvent of dichloromethane and absolute ethanol (ratio 3:1) for recrystallization to obtain 39.0 g of solid, with a yield of 96%.
[0049]After testing: the total yield of resolution: 44.4%; the optical degree is 99.0% (HPLC).
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com