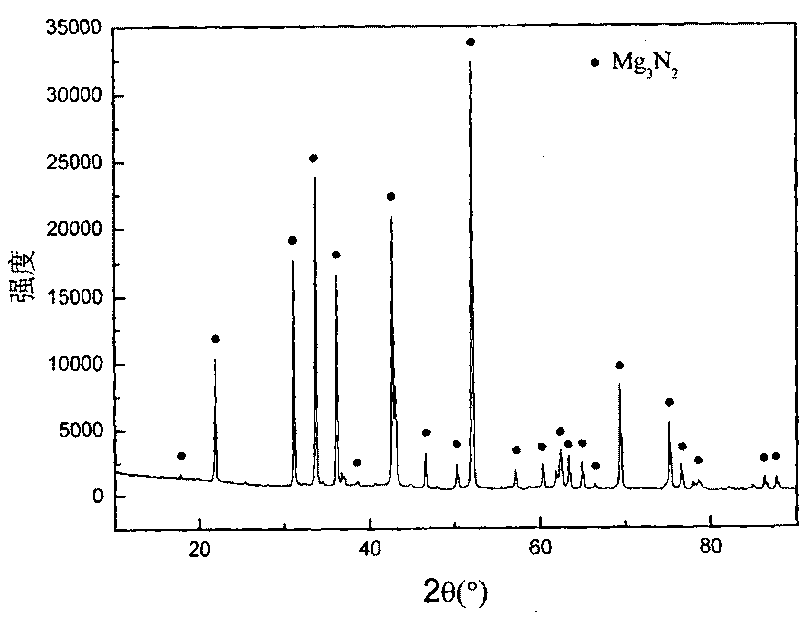
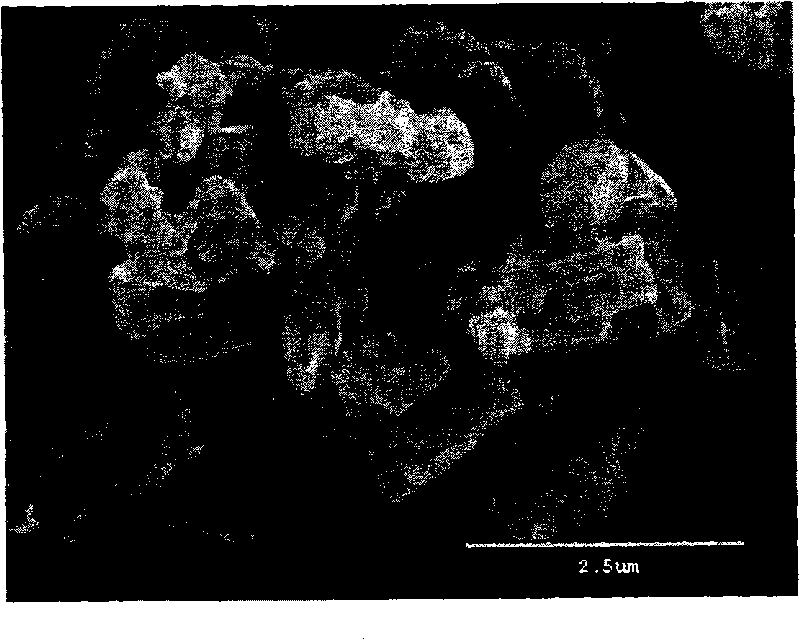
Method for synthesizing ultra-fine azotized magnesium powder with combustion
A magnesium nitride and powder technology, which is applied in the field of preparing ultrafine magnesium nitride powder by a self-propagating combustion synthesis method, can solve the problems of high nitrogen pressure, fast reaction speed, unfavorable products, etc., achieves low cost, ensures uniformity and The effect of purity and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Take Mg powder with an average particle size of 10 μm and put it directly into a porous graphite crucible covered with carbon felt at the bottom, so that the bulk density is about 1.0 g / cm 3 , put a spiral tungsten wire on the upper layer of the material, the diameter of the tungsten wire is 0.5mm, and put them together into the combustion synthesis reactor; after vacuuming, fill high-purity nitrogen, ammonia and high-purity Ar gas mixed gas, the total pressure reaches 0.5MPa, of which the partial pressure of ammonia gas is 0.2MPa, and the partial pressure of Ar gas is 0.1MPa; the spiral tungsten wire is passed with a pulse current of 10-30A for 5-10s to generate heat. The tungsten wire induces the raw material Mg powder to undergo a self-propagating combustion synthesis reaction. The highest combustion temperature reached during the reaction process is 1156°C. The combustion reaction lasts for 30 minutes. After the reaction is completed and cooled to room temperature, t...
Embodiment 2
[0031] Take Mg powder with an average particle size of 100 μm and put it directly into a porous graphite crucible covered with carbon felt at the bottom, so that the bulk density is about 2.5g / cm 3, put a spiral tungsten wire on the upper layer of the material, the diameter of the tungsten wire is 0.5mm, and put them together into the combustion synthesis reactor; after vacuuming, fill the mixture of high-purity nitrogen and high-purity Ar gas from the bottom of the reactor gas, the total pressure reaches 5.0MPa, and the partial pressure of Ar gas is 1.0MPa; the spiral tungsten wire is passed with a pulse current of 10-30A, and the duration is 5-10s, so that the heating tungsten wire induces the raw material Mg powder to undergo a self-propagating combustion synthesis reaction , the highest combustion temperature reached during the reaction is 1368°C, and the combustion reaction lasts for 30 minutes. After the reaction is completed, after cooling to room temperature, the gas in...
Embodiment 3
[0033] Take Mg powder with an average particle size of 50 μm, and put it directly into a porous graphite crucible covered with carbon felt at the bottom, so that the bulk density is about 1.5g / cm 3 , place carbon paper on the upper layer of the material, and put them together into the combustion synthesis reactor; after vacuuming, fill high-purity N from the bottom of the reactor 2 gas, high purity H 2 gas and Ar gas mixture, the total pressure reaches 4.0MPa, the Ar gas partial pressure is 1.0MPa, high-purity H 2 The gas partial pressure is 0.5MPa; the carbon paper is passed a pulse current of 10-30A for a duration of 5-10s, so that the heating carbon paper induces the raw material Mg powder to undergo a self-propagating combustion synthesis reaction, and the highest combustion temperature reached during the reaction process The temperature is 1297°C, and the combustion reaction lasts for 30 minutes. After the reaction is completed and cooled to room temperature, the gas in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com