Method for preparing hydroxyl aromatic aldehyde and aromatic ketone
A technology for aromatic aldehydes and alcoholic hydroxyl groups, which is applied in the oxidation preparation of carbonyl compounds, chemical instruments and methods, organic compounds/hydrides/coordination complex catalysts, etc., and can solve the problems of high toxicity and low yield of chromium-containing reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
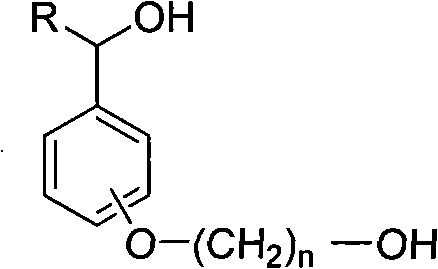
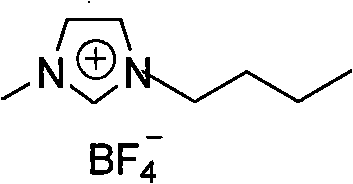
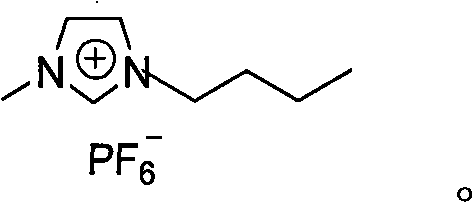
[0012] Dissolve 3.36 g (20 mmol) of 4-(2-hydroxyethoxy) benzyl alcohol and 2,2,6,6-tetramethylpiperidine nitroxide (0.2 mmol) in a 100 ml single-necked bottle In 20 ml of 1-butyl-3-methylimidazolium hexafluorophosphate ionic liquid, add sodium bromide (20 mg, 0.2 mmol) and 20 ml of potassium carbonate (0.35 g, 2.5 mmol) / sodium bicarbonate (2.1 g, 25 mmol) buffer solution, pH=8.6, finally add chlorosuccinimide (2.93 g, 22 mmol), the mixture is stirred and reacted at room temperature, follow the reaction with thin layer chromatography (TLC), After completion of the reaction, the product was extracted with ether (3×30 ml), the organic phases were combined, dried with anhydrous sodium sulfate for 2 hours, and concentrated, and the resulting crude product was subjected to silica gel column chromatography (eluent was n-hexane: ethyl acetate = 5:1), separated and obtained 2.32 grams of light yellow oily product 4-(2-hydroxyethoxy)benzaldehyde, with a yield of 70%.
[0013]
[001...
Embodiment 2
[0018] Dissolve 3.36 g (20 mmol) of 4-(2-hydroxyethoxy) benzyl alcohol and 2,2,6,6-tetramethylpiperidine nitroxide (0.2 mmol) in a 250 ml single-necked bottle In 60 ml of 1-butyl-3-methylimidazolium hexafluorophosphate ionic liquid, add sodium bromide (20 mg, 0.2 mmol) and 60 ml of potassium carbonate (0.35 g, 2.5 mmol) / sodium bicarbonate (2.1 g, 25 mmol) buffer solution, pH=8.6, finally add chlorosuccinimide (2.93 g, 22 mmol), the mixture is stirred and reacted at room temperature, follow the reaction with thin layer chromatography (TLC), After completion of the reaction, the product was extracted with ether (3×50 ml), the organic phases were combined, dried over anhydrous sodium sulfate for 2 hours, and concentrated, and the resulting crude product was subjected to silica gel column chromatography (eluent was n-hexane: ethyl acetate = 5:1), isolated and obtained 2.82 g of light yellow oily product 4-(2-hydroxyethoxy)benzaldehyde, with a yield of 85%.
Embodiment 3
[0020] The reaction steps are the same as in Example 2, except that the consumption of 2,2,6,6-tetramethylpiperidine nitrogen oxide catalyst is 0.1 molar equivalent of raw material, and the crude product is purified to obtain product 4-(2-hydroxyl Ethoxy) benzaldehyde 2.82 grams, yield is 85%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap