Polypeptide combinative with PKR kinase structure field specificity and uses thereof
A kinase domain and specific technology, applied in peptides, material inspection products, biological tests, etc., can solve the problems of no PKR inhibitors and PKR polypeptide inhibitors reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
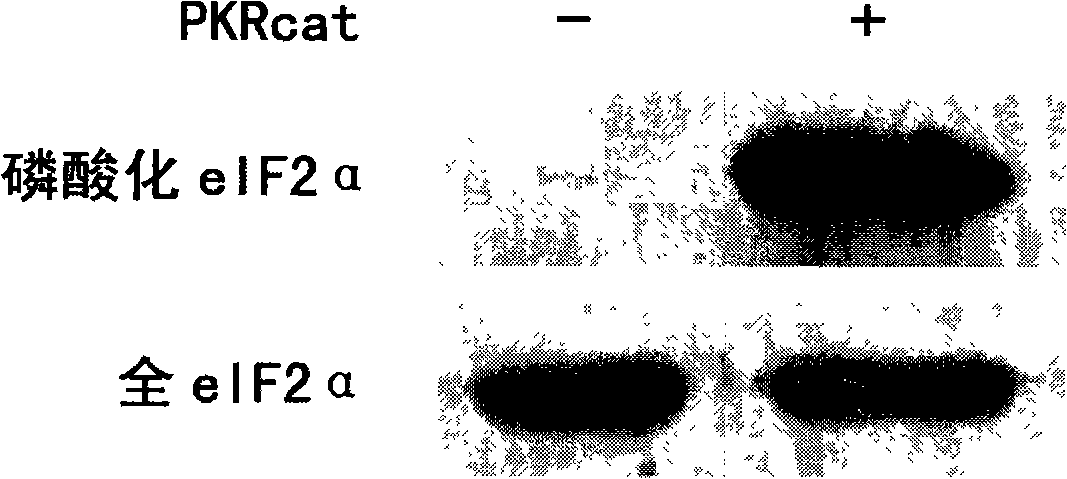
[0026] Embodiment 1: recombinant PKR cat Preliminary study on the expression, purification and activity of
[0027] 1. Recombinant PKR cat Plasmid construction
[0028] Use the existing pET28a-PKR template, the following PCR primers and Pfu polymerase to PCR amplify PKR cat :
[0029] Forward primer: 5′-CATGCCATGGGCGACATGAAAGAAACAAAGTATACTG-3′
[0030] Reverse primer: 5′-GGCTCTCGAGACATGTGTGTCGTTCATTTTTCTC-3′
[0031] The amplified DNA fragment was digested with Nco I / Xho I and cloned into plasmid pET28a (Novagen). The recombinant plasmid thus constructed was named pET28a-PKR cat And introduced Escherichia coli BL21. Recombinant plasmid pET28a-PKR cat Allowed in mature PKR cat Recombinant PKR with an additional methionine residue at the N-terminus and an additional 6 histidines at the C-terminus cat expression.
[0032] 2. Recombinant PKR cat purification of
[0033] ①. Directly containing pET28a-PKR cat BL21 in 5 mL LB medium (50 μg / ml kanamycin), 37 ° C, 250 rpm...
Embodiment 2
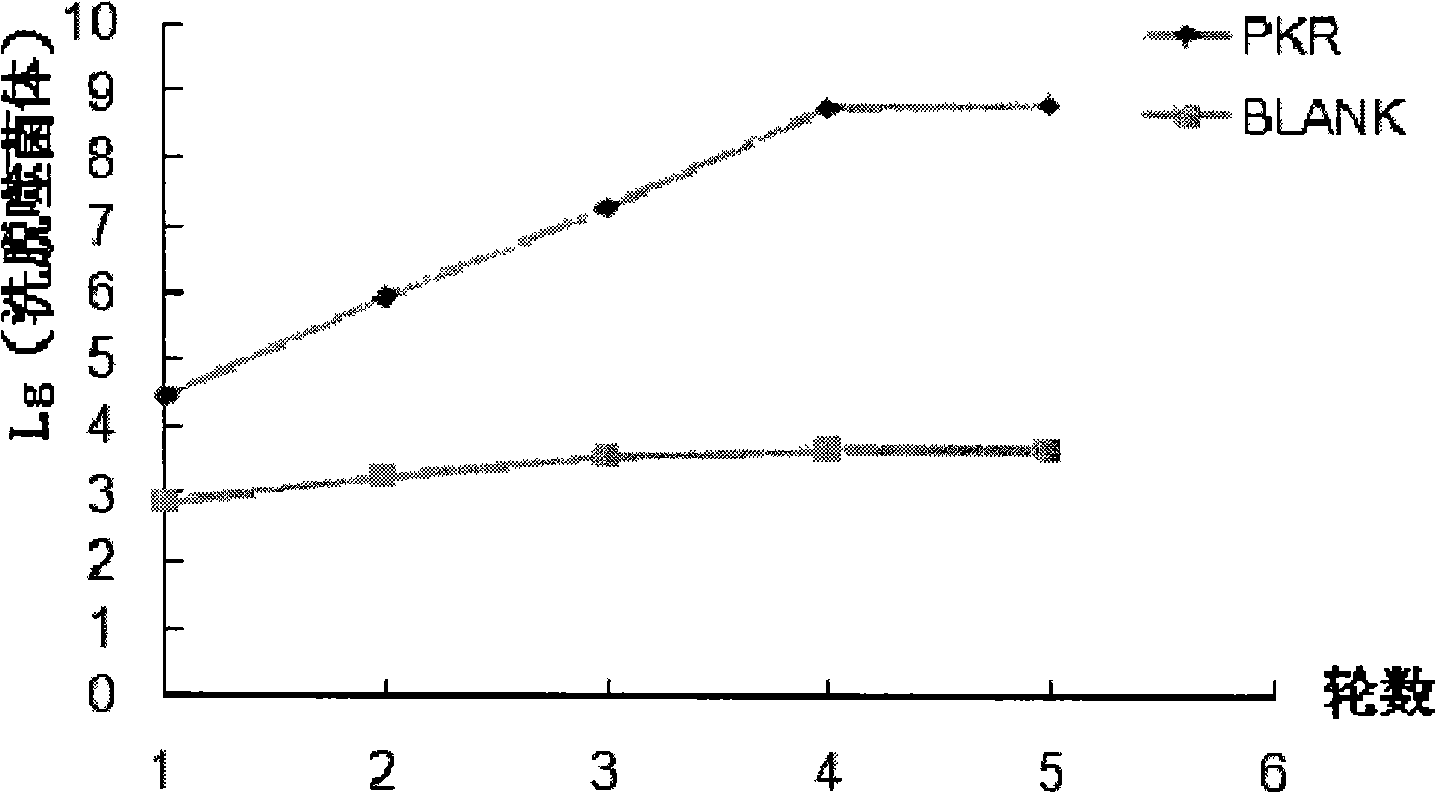
[0055] Example 2: Obtaining recombinant phage that can specifically bind to PKRcat through five rounds of screening
[0056] 1. The first round of screening and elution:
[0057] ①. Use NaHCO 3 solution dilution PKR cat Protein to 100 μg / mL solution, requires NaHCO 3 The final concentration was 0.1M. Add 100 μL of this solution to one well of a 96-well plate, and coat overnight at 4°C.
[0058] ②. Add 150 μL of freshly prepared TBS (BTBS) solution containing 0.5% (w / v) BSA to the protein-coated wells in the previous step, and place on a rocking platform at room temperature for 1 hour.
[0059] ③. At the same time, the phage (10 11 pfu) was added to 100 μL BTBS, added to an empty well, and placed on a rocking platform at room temperature for 1 hour, the purpose was to absorb the phage that could bind non-specifically to the ELISA plate.
[0060] ④. Pour off the protein / BTBS solution and wash 6 times with 200 μL TBST (TBS containing 0.1% Tween, pH 7.4), taking care not to...
Embodiment 3
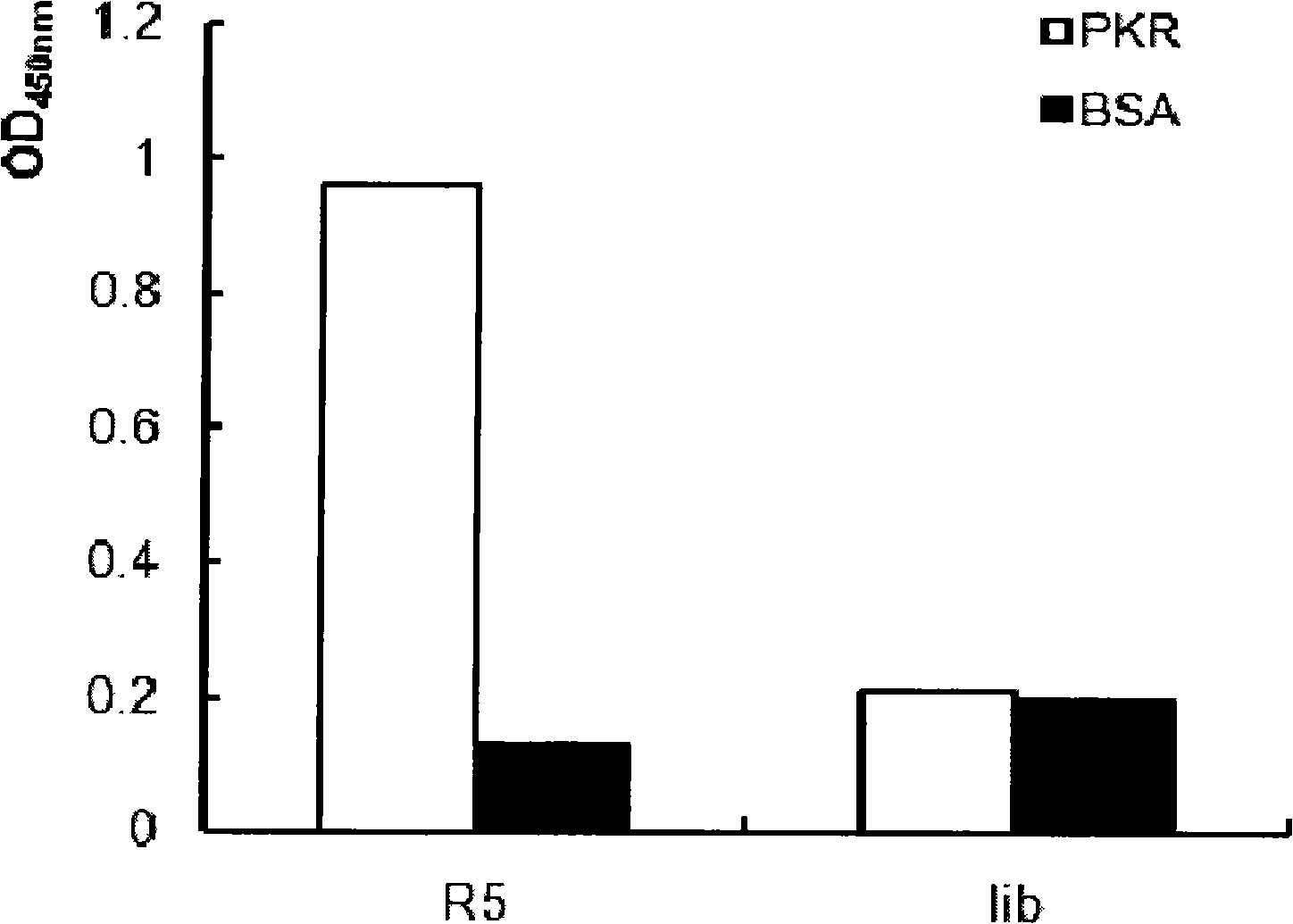
[0087] Embodiment 3: ELISA detection screened phage to PKRcat and control binding ability
[0088] ①. Use NaHCO 3 Solution Dilute protein to 100 μg / mL solution, call for NaHCO 3 The final concentration was 0.1M. Add 100 μL of this solution to two wells of a 96-well plate, and fix overnight at 4°C. At the same time, BSA was added to the other two wells as a control according to the same method.
[0089] ②. Add 100 μL of 3% BSA to each well to block the part of unbound protein, and place on a rocking platform at room temperature for 1 hour.
[0090] ③. According to the measured concentration of the phage eluted in the fifth round, dilute the phage with TBST, add 100 μL to each well, and the phage is about 10 9 -10 10 . Place on rocking platform at room temperature for 1 hour.
[0091] ④. Each well was washed 6 times with 200 μl TBST.
[0092] ⑤. HRP-anti-M13 antibody (Pharmacia Biotech Company) was diluted 1:5000 with TBST, added 100 μL to each well, and placed on a rock...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com